Trazodone: Difference between revisions
No edit summary |
No edit summary |
||
| (3 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{ | {{Infobox drug | ||
[[File:Trazodone. | | Watchedfields = verified | ||
| verifiedrevid = 457287206 | |||
| image = Trazodone.svg | |||
| image_class = skin-invert-image | |||
| width = 250 | |||
| image2 = Trazodone-from-HCl-xtal-Mercury-3D-balls.png | |||
| image_class2 = bg-transparent | |||
| width2 = 250 | |||
<!--Clinical data--> | |||
| tradename = Desyrel, Trittico, others | |||
| Drugs.com = {{drugs.com|monograph|trazodone-hydrochloride}} | |||
| MedlinePlus = a681038 | |||
| DailyMedID = Trazodone | |||
| dependency_liability = Low | |||
| addiction_liability = Low-Moderate | |||
| routes_of_administration = [[Oral administration|By mouth]] | |||
| ATC_prefix = N06 | |||
| ATC_suffix = AX05 | |||
<!-- Legal status --> | |||
| legal_AU = S4 | |||
| legal_AU_comment = | |||
| legal_BR = C1 | |||
| legal_BR_comment = | |||
| legal_CA = Rx-only | |||
| legal_CA_comment = | |||
| legal_DE = | |||
| legal_DE_comment = | |||
| legal_NZ = | |||
| legal_NZ_comment = | |||
| legal_UK = POM | |||
| legal_UK_comment = | |||
| legal_US = Rx-only | |||
| legal_US_comment = | |||
| legal_EU = | |||
| legal_EU_comment = | |||
| legal_UN = | |||
| legal_UN_comment = | |||
| legal_status = Rx-only | |||
<!--Pharmacokinetic data--> | |||
| bioavailability = [[Oral administration|By mouth]]: 65% | |||
| protein_bound = 89–95% | |||
| metabolism = [[Liver]] ([[CYP3A4]], [[CYP2D6]], [[CYP1A2]]?) | |||
| metabolites = {{abbrlink|mCPP|meta-Chlorophenylpiperazine}} | |||
| onset = [[Oral administration|By mouth]]: 1 hour ([[Tmax (pharmacology)|{{abbr|T<sub>max</sub>|Time to peak concentrations}}]]) | |||
| elimination_half-life = • Trazodone ({{abbr|IR|immediate-release}}): 4–15 hours<br />• Trazodone ({{abbr|ER|extended-release}}): 9–13 hours<br />• {{abbrlink|mCPP|meta-Chlorophenylpiperazine}}: 3–16 hours | |||
| excretion = [[Urine]]: 70–75%<br />[[Feces]]: 21% | |||
<!--Identifiers--> | |||
| index2_label = as HCl | |||
| CAS_number = 19794-93-5 | |||
| PubChem = 5533 | |||
| IUPHAR_ligand = 213 | |||
| DrugBank = DB00656 | |||
| ChemSpiderID = 5332 | |||
| UNII = YBK48BXK30 | |||
| KEGG = D08626 | |||
| KEGG2 = D00820 | |||
| ChEBI = 9654 | |||
| ChEMBL = 621 | |||
| synonyms = AF-1161 | |||
<!--Chemical data--> | |||
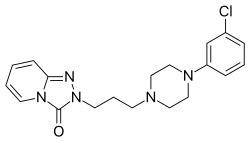
| IUPAC_name = 2-<nowiki/>{3-[4-(3-Chlorophenyl)piperazin-1-yl]propyl}[1,2,4]triazolo[4,3-''a'']pyridin-3(2''H'')-one | |||
| C=19 | H=22 | Cl=1 | N=5 | O=1 | |||
| SMILES = Clc4cccc(N3CCN(CCCN1/N=C2/C=C\C=C/N2C1=O)CC3)c4 | |||
| StdInChI = 1S/C19H22ClN5O/c20-16-5-3-6-17(15-16)23-13-11-22(12-14-23)8-4-10-25-19(26)24-9-2-1-7-18(24)21-25/h1-3,5-7,9,15H,4,8,10-14H2 | |||
| StdInChIKey = PHLBKPHSAVXXEF-UHFFFAOYSA-N | |||
<!--Physical data--> | |||
| melting_point = 87 | |||
}} | |||
[[File:Trazodone-from-HCl-xtal-Mercury-3D-balls.png|3D model of Trazodone|thumb]] | |||
Trazodone is a serotoninergic modulating antidepressant that is used in therapy of [[depression]], aggressive behavior and panic disorder. | Trazodone is a serotoninergic modulating antidepressant that is used in therapy of [[depression]], aggressive behavior and panic disorder. | ||
{{livtox}} | {{livtox}} | ||
Latest revision as of 13:35, 22 March 2025
| Trazodone | |
|---|---|
| |
| INN | |
| Drug class | |
| Routes of administration | By mouth |
| Pregnancy category | |
| Bioavailability | By mouth: 65% |
| Metabolism | Liver (CYP3A4, CYP2D6, CYP1A2?) |
| Elimination half-life | • Trazodone (IR
): 4–15 hours ): 9–13 hours
|
| Excretion | Urine: 70–75% Feces: 21% |
| Legal status | Rx-only |
| CAS Number | 19794-93-5 |
| PubChem | 5533 |
| DrugBank | DB00656 |
| ChemSpider | 5332 |
| KEGG | D08626 |

Trazodone is a serotoninergic modulating antidepressant that is used in therapy of depression, aggressive behavior and panic disorder.
Liver safety of Trazodone[edit]
Trazodone therapy can be associated with transient, usually asymptomatic elevations in serum aminotransferase levels and has been linked to rare instances of clinically apparent acute liver injury.
Mechanism of action of Trazodone[edit]
Trazodone (traz' oh done) is a triazolopyridine derivative whose mechanism of action is believed to be inhibition of serotonin and norepinephrine reuptake, which results in increased levels and activity of these neurotransmitters.
FDA approval information for Trazodone[edit]
Trazodone was approved for use in major depressive disorder in the United States in 1981 and remains in wide use, with more than 15 million prescriptions being filled yearly.
Dosage and administration for Trazodone[edit]
Trazodone is also used off-label for control of aggressive behavior and for panic disorder. Trazodone is available in tablets of 50, 75, 100, 150 and 300 mg in several generic forms.
Dosage and administration for Trazodone[edit]
The recommended dosage for depression in adults is 150 in divided doses that can be increased in 50 mg amounts to a maximum of 600 mg daily. An extended release formulation is also available in 150 mg tablets (Oleptro) which is given once daily.
Side effects of Trazodone[edit]
Common side effects of trazodone are drowsiness, fatigue, dizziness, headache, dry mouth, blurred vision, nausea, decreased libido, increased appetite and weight gain. The following are antidepressant subclasses and drugs
MAO Inhibitors Isocarboxazid, Phenelzine, Tranylcypromine
SNRIs Duloxetine, Levomilnacipran, Venlafaxine
SSRIs Citalopram, Escitalopram, Fluoxetine, Fluvoxamine, Paroxetine, Sertraline, Vilazodone, Vortioxetine
Tricyclics Amitriptyline, Amoxapine, Clomipramine, Desipramine, Doxepin, Imipramine, Nortriptyline, Protriptyline, Trimipramine
Miscellaneous Bupropion, Flibanserin, Mirtazapine, Nefazodone, Trazodone
| Medical uses |
|---|
|
The following are antidepressant subclasses and drugs MAO Inhibitors Isocarboxazid, Phenelzine, Tranylcypromine SNRIs Duloxetine, Levomilnacipran, Venlafaxine SSRIs Citalopram, Escitalopram, Fluoxetine, Fluvoxamine, Paroxetine, Sertraline, Vilazodone, Vortioxetine Tricyclics Amitriptyline, Amoxapine, Clomipramine, Desipramine, Doxepin, Imipramine, Nortriptyline, Protriptyline, Trimipramine Miscellaneous Bupropion, Flibanserin, Mirtazapine, Nefazodone, Trazodone |