Miproxifene: Difference between revisions
From WikiMD's Wellness Encyclopedia
CSV import |
CSV import |
||
| Line 72: | Line 72: | ||
{{antineoplastic-drug-stub}} | {{antineoplastic-drug-stub}} | ||
{{dictionary-stub1}} | {{dictionary-stub1}} | ||
<gallery> | |||
File:Miproxifene.svg|Miproxifene | |||
</gallery> | |||
<gallery> | <gallery> | ||
File:Miproxifene.svg|Miproxifene | File:Miproxifene.svg|Miproxifene | ||
</gallery> | </gallery> | ||
Revision as of 01:41, 20 February 2025
| Miproxifene | |
|---|---|
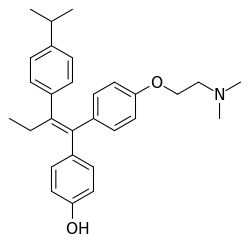
| |
| INN | |
| Drug class | |
| Routes of administration | |
| Pregnancy category | |
| Bioavailability | |
| Metabolism | |
| Elimination half-life | |
| Excretion | |
| Legal status | |
| CAS Number | 129612-87-9 |
| PubChem | 3037015 |
| DrugBank | |
| ChemSpider | 2300875 |
| KEGG | |
Miproxifene (INN) (former developmental code name DP-TAT-59) is a nonsteroidal selective estrogen receptor modulator (SERM) of the triphenylethylene group that was never marketed.<ref name="AdisInsight">http://webcache.googleusercontent.com/search?q=cache:FyhxGXFiLogJ:adisinsight.springer.com/drugs/800000796+&cd=1&hl=en&ct=clnk&gl=us</ref><ref name="StellaBorchardt2007">,
Prodrugs: Challenges and Rewards. online version, Springer Science & Business Media, ISBN 978-0-387-49782-2, Pages: 168–169,</ref> It is a derivative of afimoxifene (4-hydroxytamoxifen) in which an additional 4-isopropyl group is present in the β-phenyl ring.<ref name="OettelSchillinger2012">, Estrogens and Antiestrogens I: Physiology and Mechanisms of Action of Estrogens and Antiestrogens. online version, Springer Science & Business Media, ISBN 978-3-642-58616-3, Pages: 58–60,</ref> The drug has been found to be 3- to 10-fold more potent than tamoxifen in inhibiting breast cancer cell growth in in vitro models.<ref name="AdisInsight" /><ref name="KelloffHawk2008">, Cancer Chemoprevention: Volume 2: Strategies for Cancer Chemoprevention. online version, Springer, ISBN 978-1-59259-768-0, Pages: 251–,</ref><ref name="OttowWeinmann2008">, Nuclear Receptors as Drug Targets. online version, John Wiley & Sons, ISBN 978-3-527-62330-3, Pages: 90–,</ref> Miproxifene is the active metabolite of miproxifene phosphate (TAT-59), a phosphate ester and prodrug of miproxifene that was developed to improve its water solubility.<ref name="AdisInsight" /><ref name="StellaBorchardt2007" /><ref name="StromgaardKrogsgaard-Larsen2016">, Textbook of Drug Design and Discovery, Fifth Edition. online version, CRC Press, ISBN 978-1-4987-0279-9, Pages: 162–,</ref><ref name="YangYeh2013">, Enzyme Technologies: Pluripotent Players in Discovering Therapeutic Agent. online version, Wiley, ISBN 978-1-118-73989-1, Pages: 166–,</ref> Miproxifene phosphate was under development for the treatment of breast cancer and reached phase III clinical trials for this indication but development was discontinued.<ref name="AdisInsight" />
References
<references group="" responsive="0"></references>





