Androstanolone propionate: Difference between revisions
CSV import |
CSV import Tags: mobile edit mobile web edit |
||
| Line 4: | Line 4: | ||
File:Dihydrotestosterone_propionate_molecule_ball.png|Ball-and-stick model of Dihydrotestosterone propionate | File:Dihydrotestosterone_propionate_molecule_ball.png|Ball-and-stick model of Dihydrotestosterone propionate | ||
</gallery> | </gallery> | ||
== Androstanolone Propionate == | |||
'''Androstanolone propionate''' is a synthetic [[androgen]] and [[anabolic steroid]] (AAS) that is a derivative of [[dihydrotestosterone]] (DHT). It is an ester of [[androstanolone]], also known as [[dihydrotestosterone]], and is used in the treatment of certain medical conditions related to androgen deficiency. | |||
== Chemical Structure and Properties == | |||
Androstanolone propionate is the C17_ propionate ester of androstanolone. The chemical structure of androstanolone propionate is characterized by the presence of a propionate group attached to the 17-beta hydroxyl group of the androstanolone molecule. This modification increases the lipophilicity of the compound, allowing it to be administered via intramuscular injection and providing a sustained release of the active hormone. | |||
== Mechanism of Action == | |||
As a potent androgen, androstanolone propionate exerts its effects by binding to the [[androgen receptor]] (AR) in target tissues. Upon binding, the hormone-receptor complex translocates to the cell nucleus, where it binds to specific [[DNA]] sequences known as androgen response elements (AREs). This interaction modulates the transcription of target genes, leading to the expression of proteins that mediate the androgenic and anabolic effects of the hormone. | |||
== Medical Uses == | |||
Androstanolone propionate has been used in the treatment of conditions associated with androgen deficiency, such as [[hypogonadism]] in males. It may also be used in certain cases of [[delayed puberty]] and to promote [[muscle growth]] in patients with muscle wasting conditions. However, its use is limited due to the availability of other androgenic compounds with more favorable pharmacokinetic profiles. | |||
== Pharmacokinetics == | |||
The pharmacokinetics of androstanolone propionate are characterized by its esterification, which prolongs the duration of action compared to non-esterified androstanolone. After intramuscular injection, the ester is slowly hydrolyzed to release free androstanolone, which then exerts its biological effects. The half-life of androstanolone propionate is longer than that of free androstanolone, allowing for less frequent dosing. | |||
== Side Effects == | |||
As with other androgens, the use of androstanolone propionate can lead to a range of side effects. These may include [[acne]], [[hirsutism]], [[alopecia]], and [[virilization]] in females. In males, excessive use can lead to [[testicular atrophy]], [[gynecomastia]], and [[infertility]]. Long-term use of high doses can also have adverse effects on the [[cardiovascular system]]. | |||
== Related Pages == | |||
* [[Androgen]] | |||
* [[Anabolic steroid]] | |||
* [[Dihydrotestosterone]] | |||
* [[Hypogonadism]] | |||
* [[Testosterone]] | |||
{{Androgens}} | |||
{{Anabolic steroids}} | |||
[[Category:Androgens and anabolic steroids]] | |||
[[Category:Propionate esters]] | |||
Latest revision as of 00:37, 19 February 2025
Androstanolone propionate[edit]
-
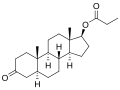
Chemical structure of Androstanolone propionate
-
Ball-and-stick model of Dihydrotestosterone propionate
Androstanolone Propionate[edit]
Androstanolone propionate is a synthetic androgen and anabolic steroid (AAS) that is a derivative of dihydrotestosterone (DHT). It is an ester of androstanolone, also known as dihydrotestosterone, and is used in the treatment of certain medical conditions related to androgen deficiency.
Chemical Structure and Properties[edit]
Androstanolone propionate is the C17_ propionate ester of androstanolone. The chemical structure of androstanolone propionate is characterized by the presence of a propionate group attached to the 17-beta hydroxyl group of the androstanolone molecule. This modification increases the lipophilicity of the compound, allowing it to be administered via intramuscular injection and providing a sustained release of the active hormone.
Mechanism of Action[edit]
As a potent androgen, androstanolone propionate exerts its effects by binding to the androgen receptor (AR) in target tissues. Upon binding, the hormone-receptor complex translocates to the cell nucleus, where it binds to specific DNA sequences known as androgen response elements (AREs). This interaction modulates the transcription of target genes, leading to the expression of proteins that mediate the androgenic and anabolic effects of the hormone.
Medical Uses[edit]
Androstanolone propionate has been used in the treatment of conditions associated with androgen deficiency, such as hypogonadism in males. It may also be used in certain cases of delayed puberty and to promote muscle growth in patients with muscle wasting conditions. However, its use is limited due to the availability of other androgenic compounds with more favorable pharmacokinetic profiles.
Pharmacokinetics[edit]
The pharmacokinetics of androstanolone propionate are characterized by its esterification, which prolongs the duration of action compared to non-esterified androstanolone. After intramuscular injection, the ester is slowly hydrolyzed to release free androstanolone, which then exerts its biological effects. The half-life of androstanolone propionate is longer than that of free androstanolone, allowing for less frequent dosing.
Side Effects[edit]
As with other androgens, the use of androstanolone propionate can lead to a range of side effects. These may include acne, hirsutism, alopecia, and virilization in females. In males, excessive use can lead to testicular atrophy, gynecomastia, and infertility. Long-term use of high doses can also have adverse effects on the cardiovascular system.
Related Pages[edit]
| Androgens and antiandrogens | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|