Mitozolomide: Difference between revisions
From WikiMD's Wellness Encyclopedia
CSV import |
CSV import |
||
| Line 66: | Line 66: | ||
{{antineoplastic-drug-stub}} | {{antineoplastic-drug-stub}} | ||
{{dictionary-stub1}} | {{dictionary-stub1}} | ||
<gallery> | |||
File:Mitozolomide.svg|Mitozolomide | |||
</gallery> | |||
Latest revision as of 01:45, 20 February 2025
| Mitozolomide | |
|---|---|

| |
| INN | |
| Drug class | |
| Routes of administration | |
| Pregnancy category | |
| Bioavailability | |
| Metabolism | |
| Elimination half-life | |
| Excretion | |
| Legal status | |
| CAS Number | 85622-95-3 |
| PubChem | 71766 |
| DrugBank | |
| ChemSpider | 64805 |
| KEGG | |
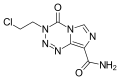
Mitozolomide (INN) is an antineoplastic. It is an imidazotetrazine derivative.
Development of mitozolomide was discontinued during Phase II clinical trials after it was found to cause severe and unpredictable bone marrow suppression.<ref>,
Enhancing hemopoietic drug resistance: a rationale for reconsidering the clinical use of mitozolomide, Cancer Gene Ther, Vol. 7(Issue: 2), pp. 233–9, DOI: 10.1038/sj.cgt.7700120, PMID: 10770631, Full text,</ref> Temozolomide, which has been in clinical use since 1999, is a less toxic analogue of mitozolomide.<ref>, Phase I trial of temozolomide (CCRG 81045: M&B 39831: NSC 362856), Br J Cancer, Vol. 65(Issue: 2), pp. 287–91, DOI: 10.1038/bjc.1992.57, PMID: 1739631, PMC: 1977719,</ref>
References[edit]
<references/>