Pelger–Huët anomaly: Difference between revisions
CSV import Tags: mobile edit mobile web edit |
No edit summary |
||
| Line 1: | Line 1: | ||
{{Short description|Genetic blood disorder with abnormal neutrophil nuclei}} | |||
{{Infobox medical condition (new) | {{Infobox medical condition (new) | ||
| name = Pelger–Huët anomaly | | name = Pelger–Huët anomaly | ||
| image = Hypogranular neutrophil with a pseudo-Pelger-Huet nucleus in MDS.jpg | | image = Hypogranular neutrophil with a pseudo-Pelger-Huet nucleus in MDS.jpg | ||
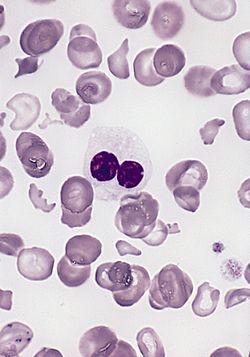
| caption = [[ | | caption = [[Blood smear]] of a patient with [[myelodysplastic syndrome]]: red blood cells showing marked [[poikilocytosis]] (partly post-[[splenectomy]]) and a hypogranular [[neutrophil]] with a pseudo-Pelger–Huët nucleus. | ||
| pronounce | | pronounce = {{IPAc-en|US|ˈ|p|ɛ|l|g|ər|_|ˈ|h|uː|ɛ|t}}<br>{{IPA-nl|ˈpɛlɣər ˈɦuːɛt}} | ||
| specialty | | specialty = [[Hematology]], [[Medical genetics]] | ||
| synonyms | | synonyms = PHA, Pelger anomaly, Huët anomaly | ||
| complications | | complications = Generally benign in heterozygotes; potential immune dysfunction in homozygotes | ||
| onset | | onset = Congenital | ||
| duration | | duration = Lifelong | ||
| types | | types = Congenital Pelger–Huët anomaly, Pseudo–Pelger–Huët anomaly | ||
| causes | | causes = [[Mutation]] in the [[lamin B receptor]] (LBR) gene | ||
| risks | | risks = Family history of the disorder | ||
| diagnosis | | diagnosis = [[Peripheral blood smear]], genetic testing | ||
| differential | | differential = [[Pseudo–Pelger–Huët anomaly]], [[Myelodysplastic syndrome]] | ||
| prevention | | prevention = None | ||
| treatment | | treatment = Supportive; no specific treatment needed for congenital form | ||
| medication | | medication = None | ||
| prognosis | | prognosis = Excellent for heterozygous congenital form | ||
| frequency | | frequency = Rare | ||
| deaths | | deaths = None (for congenital form) | ||
}} | }} | ||
[[Image:Autosomal dominant - en.svg|thumb|right|130px|Pelger–Huët anomaly has an autosomal dominant pattern of [[inheritance]].]] | |||
'''Pelger–Huët anomaly''' ('''PHA''') is a rare, benign [[genetic disorder]] affecting the [[white blood cell]]s, primarily the [[neutrophil]]s and [[eosinophil]]s. It is classified as a type of [[laminopathy]] and results from mutations in the [[lamin B receptor]] ('''LBR''') gene. The hallmark of PHA is the abnormal shape of the [[cell nucleus]], which typically appears as bilobed, peanut-shaped, or dumbbell-shaped, rather than the usual multi-lobed (trilobed) configuration. | |||
''' | ==History== | ||
[[ | The condition was first described in 1928 by Dutch hematologist '''Karel Pelger''', who noted the distinctive appearance of neutrophil nuclei. In 1931, '''Gauthier Jean Huët''', a Dutch pediatrician, recognized the hereditary nature of this anomaly, leading to the eponymous naming of the disorder. | ||
==Pathophysiology== | |||
The lamin B receptor gene, located on chromosome 1q42.1, encodes an inner nuclear membrane protein involved in chromatin organization and nuclear envelope stability. Mutations in this gene disrupt the terminal differentiation of neutrophils, leading to characteristic nuclear hyposegmentation. | |||
==Genetics== | |||
Pelger–Huët anomaly is inherited in an [[autosomal dominant]] pattern: | |||
* '''Heterozygotes''' exhibit typical bilobed or hyposegmented nuclei in neutrophils but are clinically asymptomatic. | |||
* '''Homozygotes''' may present with more pronounced nuclear abnormalities and can have mild hematological dysfunction, including altered neutrophil mobility and impaired chemotaxis. | |||
==Types== | |||
=== Congenital Pelger–Huët anomaly === | |||
The congenital form is inherited and considered benign. It is not associated with illness or increased susceptibility to infection in heterozygous carriers. The neutrophils in these individuals retain normal function despite abnormal nuclear morphology. | |||
=== Pseudo–Pelger–Huët anomaly === | |||
This is an acquired form and may occur in association with: | |||
* [[Myelodysplastic syndrome]] (MDS) | |||
* [[Acute myeloid leukemia]] (AML) | |||
* [[Chronic myelogenous leukemia]] (CML) | |||
* [[HIV/AIDS]] | |||
* After exposure to certain medications (e.g., chemotherapeutic agents, immunosuppressants) | |||
This form can be an indicator of serious underlying pathology and requires further investigation. | |||
== | ==Diagnosis== | ||
Diagnosis of Pelger–Huët anomaly includes: | |||
* **Peripheral blood smear**: Neutrophils with bilobed or round nuclei and dense clumped chromatin. | |||
* **Genetic testing**: Confirmation of LBR gene mutation if necessary. | |||
* **Family history**: Important to distinguish congenital from acquired forms. | |||
==Differential diagnosis== | |||
* [[Pseudo–Pelger–Huët anomaly]] due to underlying hematologic disease | |||
* [[Neutrophil dysplasia]] in myelodysplastic syndromes | |||
* [[Left shift]] in infection or stress | |||
== | ==Clinical significance== | ||
While Pelger–Huët anomaly is largely benign in congenital form, it is crucial to distinguish it from its pseudo-form to avoid misdiagnosis. Misinterpretation as immature granulocytes can lead to inappropriate clinical management. | |||
==Management== | |||
No treatment is required for the congenital form. Recognition of the anomaly prevents unnecessary diagnostic testing or treatment. | |||
For the acquired (pseudo) form, management involves addressing the underlying disease or discontinuing the causative medication. | |||
== | ==See also== | ||
* [[Laminopathy]] | |||
* [[Neutrophil]] | |||
* [[White blood cell]] | |||
* [[Hematology]] | |||
* [[Myelodysplastic syndrome]] | |||
==External links== | ==External links== | ||
* [https://omim.org/entry/169400 OMIM Entry: Pelger–Huët anomaly] | |||
* [https://www.ncbi.nlm.nih.gov/books/NBK538262/ NCBI: Pelger–Huët Anomaly Overview] | |||
{{Cytoskeletal defects}} | {{Cytoskeletal defects}} | ||
{{Abnormal clinical and laboratory findings for blood}} | {{Abnormal clinical and laboratory findings for blood}} | ||
{{nt}} | |||
{{DEFAULTSORT:Pelger-Huet anomaly}} | {{DEFAULTSORT:Pelger-Huet anomaly}} | ||
[[Category:Autosomal dominant disorders]] | [[Category:Autosomal dominant disorders]] | ||
[[Category:Cytoskeletal defects]] | [[Category:Cytoskeletal defects]] | ||
[[Category:Abnormal clinical and laboratory findings for blood]] | [[Category:Abnormal clinical and laboratory findings for blood]] | ||
Latest revision as of 03:36, 30 March 2025
Genetic blood disorder with abnormal neutrophil nuclei
| Pelger–Huët anomaly | |
|---|---|
| |
| Synonyms | PHA, Pelger anomaly, Huët anomaly |
| Pronounce | nl |
| Field | N/A |
| Symptoms | N/A |
| Complications | Generally benign in heterozygotes; potential immune dysfunction in homozygotes |
| Onset | Congenital |
| Duration | Lifelong |
| Types | Congenital Pelger–Huët anomaly, Pseudo–Pelger–Huët anomaly |
| Causes | Mutation in the lamin B receptor (LBR) gene |
| Risks | Family history of the disorder |
| Diagnosis | Peripheral blood smear, genetic testing |
| Differential diagnosis | Pseudo–Pelger–Huët anomaly, Myelodysplastic syndrome |
| Prevention | None |
| Treatment | Supportive; no specific treatment needed for congenital form |
| Medication | None |
| Prognosis | Excellent for heterozygous congenital form |
| Frequency | Rare |
| Deaths | None (for congenital form) |

Pelger–Huët anomaly (PHA) is a rare, benign genetic disorder affecting the white blood cells, primarily the neutrophils and eosinophils. It is classified as a type of laminopathy and results from mutations in the lamin B receptor (LBR) gene. The hallmark of PHA is the abnormal shape of the cell nucleus, which typically appears as bilobed, peanut-shaped, or dumbbell-shaped, rather than the usual multi-lobed (trilobed) configuration.
History[edit]
The condition was first described in 1928 by Dutch hematologist Karel Pelger, who noted the distinctive appearance of neutrophil nuclei. In 1931, Gauthier Jean Huët, a Dutch pediatrician, recognized the hereditary nature of this anomaly, leading to the eponymous naming of the disorder.
Pathophysiology[edit]
The lamin B receptor gene, located on chromosome 1q42.1, encodes an inner nuclear membrane protein involved in chromatin organization and nuclear envelope stability. Mutations in this gene disrupt the terminal differentiation of neutrophils, leading to characteristic nuclear hyposegmentation.
Genetics[edit]
Pelger–Huët anomaly is inherited in an autosomal dominant pattern:
- Heterozygotes exhibit typical bilobed or hyposegmented nuclei in neutrophils but are clinically asymptomatic.
- Homozygotes may present with more pronounced nuclear abnormalities and can have mild hematological dysfunction, including altered neutrophil mobility and impaired chemotaxis.
Types[edit]
Congenital Pelger–Huët anomaly[edit]
The congenital form is inherited and considered benign. It is not associated with illness or increased susceptibility to infection in heterozygous carriers. The neutrophils in these individuals retain normal function despite abnormal nuclear morphology.
Pseudo–Pelger–Huët anomaly[edit]
This is an acquired form and may occur in association with:
- Myelodysplastic syndrome (MDS)
- Acute myeloid leukemia (AML)
- Chronic myelogenous leukemia (CML)
- HIV/AIDS
- After exposure to certain medications (e.g., chemotherapeutic agents, immunosuppressants)
This form can be an indicator of serious underlying pathology and requires further investigation.
Diagnosis[edit]
Diagnosis of Pelger–Huët anomaly includes:
- **Peripheral blood smear**: Neutrophils with bilobed or round nuclei and dense clumped chromatin.
- **Genetic testing**: Confirmation of LBR gene mutation if necessary.
- **Family history**: Important to distinguish congenital from acquired forms.
Differential diagnosis[edit]
- Pseudo–Pelger–Huët anomaly due to underlying hematologic disease
- Neutrophil dysplasia in myelodysplastic syndromes
- Left shift in infection or stress
Clinical significance[edit]
While Pelger–Huët anomaly is largely benign in congenital form, it is crucial to distinguish it from its pseudo-form to avoid misdiagnosis. Misinterpretation as immature granulocytes can lead to inappropriate clinical management.
Management[edit]
No treatment is required for the congenital form. Recognition of the anomaly prevents unnecessary diagnostic testing or treatment.
For the acquired (pseudo) form, management involves addressing the underlying disease or discontinuing the causative medication.
See also[edit]
External links[edit]
| Abnormal clinical and laboratory findings for blood tests (ICD-10 R70–R79, ICD-9 780–799) | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|