Alkyl nitrite: Difference between revisions
CSV import |
CSV import |
||
| Line 5: | Line 5: | ||
File:QuinineNitrateMechanism.png|Quinine nitrate mechanism | File:QuinineNitrateMechanism.png|Quinine nitrate mechanism | ||
</gallery> | </gallery> | ||
==Alkyl Nitrite== | |||
'''Alkyl nitrites''' are a group of chemical compounds based on the molecular structure R-ONO, where R represents an [[alkyl group]]. These compounds are esters of [[nitrous acid]] and are known for their use in medicine and as recreational drugs. Alkyl nitrites are volatile liquids at room temperature and are often used for their vasodilating effects. | |||
==Chemical Structure and Properties== | |||
Alkyl nitrites consist of an alkyl group attached to a nitrite group. The general formula is R-ONO, where R can be a methyl, ethyl, propyl, butyl, or other alkyl group. These compounds are characterized by their distinctive fruity odor and their ability to rapidly evaporate. | |||
Alkyl nitrites are highly flammable and should be handled with care. They are soluble in organic solvents but have limited solubility in water. | |||
==Pharmacological Effects== | |||
Alkyl nitrites are known for their vasodilatory effects, which means they can widen blood vessels. This property makes them useful in the treatment of certain medical conditions, such as [[angina pectoris]]. When inhaled, alkyl nitrites cause a rapid drop in blood pressure, leading to a sensation of warmth and a decrease in chest pain. | |||
The vasodilatory effect is due to the release of [[nitric oxide]] (NO) in the body, which is a potent vasodilator. This effect is short-lived, typically lasting a few minutes. | |||
==Medical Uses== | |||
In medicine, alkyl nitrites have been used to treat angina and other heart-related conditions. They are administered by inhalation, which allows for rapid onset of action. However, their use has declined with the development of more effective and longer-lasting medications. | |||
==Recreational Use== | |||
Alkyl nitrites are also used recreationally, often referred to as "poppers." They are inhaled for their euphoric effects and the sensation of warmth and relaxation they produce. The use of poppers is popular in certain subcultures and is often associated with dance clubs and parties. | |||
==Safety and Legal Status== | |||
The use of alkyl nitrites carries certain health risks, including the potential for [[methemoglobinemia]], a condition where hemoglobin is unable to effectively release oxygen to body tissues. Other side effects can include headaches, dizziness, and skin irritation. | |||
The legal status of alkyl nitrites varies by country. In some places, they are regulated as prescription medications, while in others, they are available over the counter or are banned entirely. | |||
==Related Pages== | |||
* [[Nitrous acid]] | |||
* [[Vasodilation]] | |||
* [[Nitric oxide]] | |||
* [[Methemoglobinemia]] | |||
{{Chemistry}} | |||
{{Pharmacology}} | |||
[[Category:Organic compounds]] | |||
[[Category:Vasodilators]] | |||
[[Category:Recreational drugs]] | |||
Latest revision as of 00:34, 19 February 2025
Alkyl_nitrite[edit]
-
Nitrite group 2D structure
-
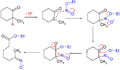
Quinine nitrite reaction
-
Quinine nitrate mechanism
Alkyl Nitrite[edit]
Alkyl nitrites are a group of chemical compounds based on the molecular structure R-ONO, where R represents an alkyl group. These compounds are esters of nitrous acid and are known for their use in medicine and as recreational drugs. Alkyl nitrites are volatile liquids at room temperature and are often used for their vasodilating effects.
Chemical Structure and Properties[edit]
Alkyl nitrites consist of an alkyl group attached to a nitrite group. The general formula is R-ONO, where R can be a methyl, ethyl, propyl, butyl, or other alkyl group. These compounds are characterized by their distinctive fruity odor and their ability to rapidly evaporate.
Alkyl nitrites are highly flammable and should be handled with care. They are soluble in organic solvents but have limited solubility in water.
Pharmacological Effects[edit]
Alkyl nitrites are known for their vasodilatory effects, which means they can widen blood vessels. This property makes them useful in the treatment of certain medical conditions, such as angina pectoris. When inhaled, alkyl nitrites cause a rapid drop in blood pressure, leading to a sensation of warmth and a decrease in chest pain.
The vasodilatory effect is due to the release of nitric oxide (NO) in the body, which is a potent vasodilator. This effect is short-lived, typically lasting a few minutes.
Medical Uses[edit]
In medicine, alkyl nitrites have been used to treat angina and other heart-related conditions. They are administered by inhalation, which allows for rapid onset of action. However, their use has declined with the development of more effective and longer-lasting medications.
Recreational Use[edit]
Alkyl nitrites are also used recreationally, often referred to as "poppers." They are inhaled for their euphoric effects and the sensation of warmth and relaxation they produce. The use of poppers is popular in certain subcultures and is often associated with dance clubs and parties.
Safety and Legal Status[edit]
The use of alkyl nitrites carries certain health risks, including the potential for methemoglobinemia, a condition where hemoglobin is unable to effectively release oxygen to body tissues. Other side effects can include headaches, dizziness, and skin irritation.
The legal status of alkyl nitrites varies by country. In some places, they are regulated as prescription medications, while in others, they are available over the counter or are banned entirely.
Related Pages[edit]
| Branches of chemistry | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| Pharmacology | ||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|