EpiVacCorona: Difference between revisions
CSV import Tags: mobile edit mobile web edit |
CSV import |
||
| Line 25: | Line 25: | ||
{{COVID-19 pandemic}} | {{COVID-19 pandemic}} | ||
{{Vaccine-stub}} | {{Vaccine-stub}} | ||
<gallery> | |||
File:EpiVacCorona_design.tif|EpiVacCorona design | |||
File:EpiVacCorona_in_illustrations.tif|EpiVacCorona in illustrations | |||
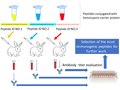
File:Rabbits_antibody_detection_scheme.tif|Rabbits antibody detection scheme | |||
</gallery> | |||
Latest revision as of 04:27, 18 February 2025
EpiVacCorona is a peptide-based vaccine against COVID-19 developed by the Vector Institute in Russia. It was authorized for emergency use in Russia in October 2020, following Phase I and II trials. As of March 2021, Phase III trials are ongoing.
Development[edit]
The Vector Institute, also known as the State Research Centre of Virology and Biotechnology, began development of EpiVacCorona in early 2020, following the global outbreak of the SARS-CoV-2 virus. The vaccine is based on synthetic peptide antigens of SARS-CoV-2 proteins, conjugated to a carrier protein and administered with an adjuvant.
Clinical Trials[edit]
EpiVacCorona underwent Phase I and II clinical trials in 2020. The trials involved a total of 100 participants and reportedly showed the vaccine to be safe and effective. However, the results have not been published in a peer-reviewed journal, leading to calls for greater transparency.
Phase III trials began in November 2020 and are expected to involve 3,000 participants. Preliminary results are expected in early 2021.
Efficacy[edit]
The Vector Institute has claimed that EpiVacCorona is 100% effective, based on the results of the Phase I and II trials. However, these claims have been met with skepticism due to the lack of published data.
Authorization[edit]
EpiVacCorona was granted emergency use authorization in Russia in October 2020, making it the second COVID-19 vaccine to be authorized in the country, after Sputnik V.
See Also[edit]
-
EpiVacCorona design
-
EpiVacCorona in illustrations
-
Rabbits antibody detection scheme