Adverse drug reaction: Difference between revisions
From WikiMD's Wellness Encyclopedia
No edit summary |
CSV import |
||
| Line 1: | Line 1: | ||
* An adverse drug reaction (ADR) refers to an unintended and harmful response to a medication. | {{Infobox medical condition | ||
| name = Adverse drug reaction | |||
| image =[[File:Drugreaction.jpg|250px]] | |||
| caption = Skin rash as a common adverse drug reaction | |||
| field = [[Pharmacology]] | |||
| synonyms = ADR, drug side effect | |||
| symptoms = [[Rash]], [[nausea]], [[vomiting]], [[diarrhea]], [[headache]], [[dizziness]], [[anaphylaxis]] | |||
| complications = [[Anaphylactic shock]], [[organ failure]], [[death]] | |||
| onset = Varies depending on the drug and individual | |||
| duration = Varies; can be temporary or permanent | |||
| causes = [[Medication]] use | |||
| risks = [[Polypharmacy]], [[allergies]], [[genetic predisposition]] | |||
| diagnosis = [[Clinical history]], [[physical examination]], [[laboratory tests]] | |||
| differential = [[Drug allergy]], [[drug intolerance]], [[drug interaction]] | |||
| prevention = [[Medication review]], [[patient education]], [[pharmacogenomics]] | |||
| treatment = Discontinuation of the drug, [[antihistamines]], [[corticosteroids]], [[supportive care]] | |||
| prognosis = Varies; can be mild to life-threatening | |||
| frequency = Common; varies by drug and population | |||
}} | |||
* An adverse drug reaction (ADR) refers to an unintended and harmful response to a medication. | |||
* It occurs when a drug, prescribed or taken as instructed, produces an undesirable or unexpected effect on the body. | * It occurs when a drug, prescribed or taken as instructed, produces an undesirable or unexpected effect on the body. | ||
* Adverse drug reactions can range from mild side effects to severe and life-threatening conditions. | * Adverse drug reactions can range from mild side effects to severe and life-threatening conditions. | ||
Latest revision as of 02:39, 4 April 2025
| Adverse drug reaction | |
|---|---|
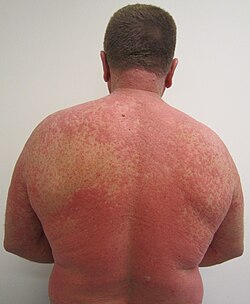
| |
| Synonyms | ADR, drug side effect |
| Pronounce | N/A |
| Specialty | N/A |
| Symptoms | Rash, nausea, vomiting, diarrhea, headache, dizziness, anaphylaxis |
| Complications | Anaphylactic shock, organ failure, death |
| Onset | Varies depending on the drug and individual |
| Duration | Varies; can be temporary or permanent |
| Types | N/A |
| Causes | Medication use |
| Risks | Polypharmacy, allergies, genetic predisposition |
| Diagnosis | Clinical history, physical examination, laboratory tests |
| Differential diagnosis | Drug allergy, drug intolerance, drug interaction |
| Prevention | Medication review, patient education, pharmacogenomics |
| Treatment | Discontinuation of the drug, antihistamines, corticosteroids, supportive care |
| Medication | N/A |
| Prognosis | Varies; can be mild to life-threatening |
| Frequency | Common; varies by drug and population |
| Deaths | N/A |
* An adverse drug reaction (ADR) refers to an unintended and harmful response to a medication.
- It occurs when a drug, prescribed or taken as instructed, produces an undesirable or unexpected effect on the body.
- Adverse drug reactions can range from mild side effects to severe and life-threatening conditions.
- Understanding ADRs is crucial for healthcare professionals, patients, and regulatory bodies to ensure the safe and effective use of medications.

Classification of Adverse Drug Reactions[edit]
- Adverse drug reactions can be classified into several categories based on different criteria.
- The most common classification system divides ADRs into the following types:
Type A (Augmented)[edit]
- Type A reactions are the most common type of adverse drug reactions and are predictable based on a drug's known pharmacological properties.
- These reactions are dose-dependent and usually related to the drug's therapeutic effect or its interaction with specific receptors or enzymes.
- Type A reactions are typically mild to moderate and include side effects such as nausea, dizziness, and headache.
Type B (Bizarre)[edit]
- Type B reactions are unpredictable and unrelated to the known pharmacological action of a drug.
- They occur infrequently and are often severe or life-threatening.
- Type B reactions are typically not dose-dependent and can vary between individuals.
- Examples of type B reactions include severe allergic reactions (anaphylaxis), drug-induced liver injury, and blood dyscrasias.
Type C (Chronic)[edit]
- Type C reactions are associated with the long-term use of a drug.
- They arise from the cumulative effects of a medication over time.
- Examples of type C reactions include drug-induced osteoporosis, tardive dyskinesia caused by long-term use of antipsychotics, and drug-induced endocrine disorders.
Type D (Delayed)[edit]
- Type D reactions occur after a significant delay following drug administration.
- These reactions can manifest weeks, months, or even years after initiating treatment.
- Examples of type D reactions include certain drug-induced cancers, such as bladder cancer associated with long-term use of certain diabetes medications.
Type E (End-of-treatment)[edit]
- Type E reactions occur when a drug is abruptly stopped or withdrawn.
- They are often related to a rebound effect or withdrawal syndrome.
- Examples include rebound hypertension after discontinuing antihypertensive medications or seizures following abrupt withdrawal of antiepileptic drugs.
Risk Factors for Adverse Drug Reactions[edit]
- Several factors can contribute to the occurrence of adverse drug reactions.
These include:
- Age: Elderly individuals and children may be more susceptible to ADRs due to differences in drug metabolism, organ function, and physiological characteristics.
- Polypharmacy: Taking multiple medications simultaneously increases the risk of drug interactions and adverse effects.
- Genetic Factors: Genetic variations can influence how an individual responds to certain drugs, potentially increasing the likelihood of ADRs.
- Pre-existing Conditions: Certain medical conditions, such as liver or kidney impairment, can affect drug metabolism and increase the risk of ADRs.
- Drug Allergies: Individuals with known drug allergies or hypersensitivity reactions may experience severe ADRs upon exposure to the specific medication.
- Concomitant Diseases: Coexisting medical conditions can interact with drugs and increase the risk of adverse effects.
Prevention and Management[edit]
- Prevention and management of adverse drug reactions involve various strategies:
- Proper Medication Selection: Healthcare professionals should carefully consider a patient's medical history, allergies, and potential drug interactions before prescribing medications.
- Patient Education: Patients should be educated about the potential side effects and risks associated with their medications. They should be encouraged to report any unusual symptoms to their healthcare provider.
- Monitoring and Surveillance: Regular monitoring of patients receiving medications can help detect and manage ADRs promptly.
- Pharmacovigilance: Pharmacovigilance programs and regulatory systems play a crucial role in monitoring and reporting ADRs at a population level. This helps identify potential safety concerns and take appropriate regulatory actions if needed.
- Adherence to Guidelines: Healthcare professionals should follow evidence-based guidelines for prescribing medications and adjust dosage or therapy based on individual patient characteristics.
- Reporting and Documentation: ADRs should be reported to the relevant regulatory authorities or pharmacovigilance programs to contribute to the overall understanding of drug safety profiles.
See Also[edit]
| Medical harm | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| Adverse drug reactions | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|