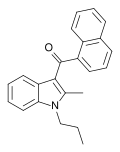
JWH-015
Chemical compound
| JWH-015 | |
|---|---|

| |
| INN | |
| Drug class | |
| Routes of administration | |
| Pregnancy category | |
| Bioavailability | |
| Metabolism | |
| Elimination half-life | |
| Excretion | |
| Legal status | |
| CAS Number | 155471-08-2 |
| PubChem | 4273754 |
| DrugBank | |
| ChemSpider | 3480676 |
| KEGG | |
JWH-015 is a chemical from the naphthoylindole family that acts as a subtype-selective cannabinoid agonist. Its affinity for CB2 receptors is 13.8 nM, while its affinity for CB1 is 383 nM, meaning that it binds almost 28 times more strongly to CB2 than to CB1.<ref name="pmid10940540">,
Influence of the N-1 alkyl chain length of cannabimimetic indoles upon CB1 and CB2)receptor binding, Drug and Alcohol Dependence, Vol. 60(Issue: 2), pp. 133–140, DOI: 10.1016/S0376-8716(99)00152-0, PMID: 10940540,</ref> However, it still displays some CB1 activity, and in some model systems can be very potent and efficacious at activating CB1 receptors,<ref name="pmid22921769">, The CB2-preferring agonist JWH015 also potently and efficaciously activates CB1 in autaptic hippocampal neurons, Pharmacological Research, Vol. 66(Issue: 5), pp. 437–442, DOI: 10.1016/j.phrs.2012.08.002, PMID: 22921769, PMC: 3601544,</ref> and therefore it is not as selective as newer drugs such as JWH-133.<ref name="pmid18289088">, Recent advances in the development of selective ligands for the cannabinoid CB(2) receptor, Current Topics in Medicinal Chemistry, 2008, Vol. 8(Issue: 3), pp. 187–204, DOI: 10.2174/156802608783498014, PMID: 18289088,</ref> It has been shown to possess immunomodulatory effects,<ref name="pmid16503355">, Cannabinoid receptor CB2 modulates the CXCL12/CXCR4-mediated chemotaxis of T lymphocytes, Molecular Immunology, Vol. 43(Issue: 14), pp. 2169–2179, DOI: 10.1016/j.molimm.2006.01.005, PMID: 16503355,</ref><ref name="pmid18178718">, CB2 cannabinoid receptor agonist JWH-015 modulates human monocyte migration through defined intracellular signaling pathways, American Journal of Physiology. Heart and Circulatory Physiology, Vol. 294(Issue: 3), pp. H1145–H1155, DOI: 10.1152/ajpheart.01328.2007, PMID: 18178718,</ref> and CB2 agonists may be useful in the treatment of pain and inflammation.<ref name="pmid1352348">, Prescribing and use of benzodiazepines: an epidemiologic perspective, Journal of Psychoactive Drugs, 1992, Vol. 24(Issue: 1), pp. 63–64, DOI: 10.1080/02791072.1992.10471620, PMID: 1352348,</ref><ref name="pmid17413917">, Spinal cannabinoid receptor type 2 activation reduces hypersensitivity and spinal cord glial activation after paw incision, Anesthesiology, Vol. 106(Issue: 4), pp. 787–794, DOI: 10.1097/01.anes.0000264765.33673.6c, PMID: 17413917,</ref> It was discovered and named after John W. Huffman.
Metabolism
JWH-015 has been shown in vitro to be metabolized primarily by hydroxylation and N-dealkylation, and also by epoxidation of the naphthalene ring,<ref>,
Identification of in vitro metabolites of JWH-015, an aminoalkylindole agonist for the peripheral cannabinoid receptor (CB2) by HPLC-MS/MS, Analytical and Bioanalytical Chemistry, Vol. 386(Issue: 5), pp. 1345–1355, DOI: 10.1007/s00216-006-0717-6, PMID: 16955257,</ref> similar to the metabolic pathways seen for other aminoalkylindole cannabinoids such as WIN 55,212-2.<ref name="pmid12228183">, In vitro metabolism of R(+)-[2,3-dihydro-5-methyl-3-[(morpholinyl)methyl]pyrrolo [1,2,3-de]1,4-benzoxazinyl]-(1-naphthalenyl) methanone mesylate, a cannabinoid receptor agonist, Drug Metabolism and Disposition, Vol. 30(Issue: 10), pp. 1077–1086, DOI: 10.1124/dmd.30.10.1077, PMID: 12228183,</ref> Epoxidation of polycyclic aromatic hydrocarbons (see for example benzo(a)pyrene toxicity) can produce carcinogenic metabolites, although there is no evidence to show that JWH-015 or other aminoalkylindole cannabinoids are actually carcinogenic in vivo. JWH-015 may signal certain cancers to shrink through a process called apoptosis.<ref>, Inhibition of human tumour prostate PC-3 cell growth by cannabinoids R(+)-Methanandamide and JWH-015: involvement of CB2, British Journal of Cancer, Vol. 101(Issue: 6), pp. 940–950, DOI: 10.1038/sj.bjc.6605248, PMID: 19690545, PMC: 2743360,</ref>
Legal status
In the United States, all CB1 receptor agonists of the 3-(1-naphthoyl)indole class such as JWH-015 are Schedule I Controlled Substances.<ref>Template:UnitedStatesCode2</ref>
As of October 2015 JWH-015 is a controlled substance in China.<ref>
关于印发《非药用类麻醉药品和精神药品列管办法》的通知(link). {{{website}}}. China Food and Drug Administration. 27 September 2015.
Accessed 1 October 2015.
</ref>
JWH-015 has been classified under the German BtMG as Anlage II.<ref>
Stoffe gem. Anlagen zum BtMG(link). {{{website}}}.
Accessed 2024-11-23.
</ref>
References
<references group="" responsive="0"></references>
-
JWH-015
Ad. Transform your life with W8MD's Budget GLP-1 injections from $75


W8MD offers a medical weight loss program to lose weight in Philadelphia. Our physician-supervised medical weight loss provides:
- Weight loss injections in NYC (generic and brand names):
- Zepbound / Mounjaro, Wegovy / Ozempic, Saxenda
- Most insurances accepted or discounted self-pay rates. We will obtain insurance prior authorizations if needed.
- Generic GLP1 weight loss injections from $75 for the starting dose.
- Also offer prescription weight loss medications including Phentermine, Qsymia, Diethylpropion, Contrave etc.
NYC weight loss doctor appointmentsNYC weight loss doctor appointments
Start your NYC weight loss journey today at our NYC medical weight loss and Philadelphia medical weight loss clinics.
- Call 718-946-5500 to lose weight in NYC or for medical weight loss in Philadelphia 215-676-2334.
- Tags:NYC medical weight loss, Philadelphia lose weight Zepbound NYC, Budget GLP1 weight loss injections, Wegovy Philadelphia, Wegovy NYC, Philadelphia medical weight loss, Brookly weight loss and Wegovy NYC
|
WikiMD's Wellness Encyclopedia |
| Let Food Be Thy Medicine Medicine Thy Food - Hippocrates |
Medical Disclaimer: WikiMD is not a substitute for professional medical advice. The information on WikiMD is provided as an information resource only, may be incorrect, outdated or misleading, and is not to be used or relied on for any diagnostic or treatment purposes. Please consult your health care provider before making any healthcare decisions or for guidance about a specific medical condition. WikiMD expressly disclaims responsibility, and shall have no liability, for any damages, loss, injury, or liability whatsoever suffered as a result of your reliance on the information contained in this site. By visiting this site you agree to the foregoing terms and conditions, which may from time to time be changed or supplemented by WikiMD. If you do not agree to the foregoing terms and conditions, you should not enter or use this site. See full disclaimer.
Credits:Most images are courtesy of Wikimedia commons, and templates, categories Wikipedia, licensed under CC BY SA or similar.
Translate this page: - East Asian
中文,
日本,
한국어,
South Asian
हिन्दी,
தமிழ்,
తెలుగు,
Urdu,
ಕನ್ನಡ,
Southeast Asian
Indonesian,
Vietnamese,
Thai,
မြန်မာဘာသာ,
বাংলা
European
español,
Deutsch,
français,
Greek,
português do Brasil,
polski,
română,
русский,
Nederlands,
norsk,
svenska,
suomi,
Italian
Middle Eastern & African
عربى,
Turkish,
Persian,
Hebrew,
Afrikaans,
isiZulu,
Kiswahili,
Other
Bulgarian,
Hungarian,
Czech,
Swedish,
മലയാളം,
मराठी,
ਪੰਜਾਬੀ,
ગુજરાતી,
Portuguese,
Ukrainian