Tryptophan hydroxylase: Difference between revisions
CSV import |
CSV import Tags: mobile edit mobile web edit |
||
| Line 4: | Line 4: | ||
File:Trp-5ht-pathway.svg|Tryptophan to serotonin pathway | File:Trp-5ht-pathway.svg|Tryptophan to serotonin pathway | ||
</gallery> | </gallery> | ||
== Tryptophan Hydroxylase == | |||
'''Tryptophan hydroxylase''' (TPH) is an enzyme that plays a crucial role in the biosynthesis of the neurotransmitter [[serotonin]]. It is the rate-limiting enzyme in the conversion of the amino acid [[tryptophan]] to 5-hydroxytryptophan (5-HTP), which is subsequently decarboxylated to form serotonin. Tryptophan hydroxylase is a member of the aromatic amino acid hydroxylase family, which also includes [[tyrosine hydroxylase]] and [[phenylalanine hydroxylase]]. | |||
== Structure == | |||
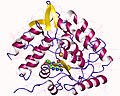
Tryptophan hydroxylase is a monomeric enzyme that requires a cofactor, [[tetrahydrobiopterin]] (BH4), for its activity. The enzyme also requires molecular [[oxygen]] and [[iron]] as a cofactor. The active site of tryptophan hydroxylase contains a non-heme iron that is essential for its catalytic function. The enzyme is composed of several domains, including a regulatory domain, a catalytic domain, and a tetramerization domain. | |||
== Isoforms == | |||
There are two isoforms of tryptophan hydroxylase, known as TPH1 and TPH2. TPH1 is primarily expressed in peripheral tissues such as the [[pineal gland]] and the [[gastrointestinal tract]], while TPH2 is predominantly found in the [[central nervous system]]. The differential expression of these isoforms allows for the regulation of serotonin synthesis in different tissues. | |||
== Function == | |||
Tryptophan hydroxylase catalyzes the hydroxylation of tryptophan to form 5-hydroxytryptophan (5-HTP). This reaction is the first and rate-limiting step in the biosynthesis of serotonin. The activity of tryptophan hydroxylase is regulated by various factors, including the availability of its substrate, tryptophan, and its cofactor, tetrahydrobiopterin. Additionally, the enzyme can be regulated by phosphorylation and by feedback inhibition from serotonin. | |||
== Clinical Significance == | |||
Alterations in tryptophan hydroxylase activity have been implicated in various disorders related to serotonin imbalance. For example, reduced activity of TPH2 has been associated with [[depression]] and [[anxiety]] disorders. Genetic polymorphisms in the TPH1 and TPH2 genes have been studied in relation to their potential impact on psychiatric conditions and response to treatment. | |||
== Related Pages == | |||
* [[Serotonin]] | |||
* [[Tryptophan]] | |||
* [[Tyrosine hydroxylase]] | |||
* [[Phenylalanine hydroxylase]] | |||
* [[Tetrahydrobiopterin]] | |||
{{Enzymes}} | |||
{{Neurotransmitters}} | |||
[[Category:Enzymes]] | |||
[[Category:Neurochemistry]] | |||
[[Category:Serotonin]] | |||
Latest revision as of 00:40, 19 February 2025
-
Tryptophan hydroxylase structure
-
Serotonin synthesis pathway
-
Tryptophan to serotonin pathway
Tryptophan Hydroxylase[edit]
Tryptophan hydroxylase (TPH) is an enzyme that plays a crucial role in the biosynthesis of the neurotransmitter serotonin. It is the rate-limiting enzyme in the conversion of the amino acid tryptophan to 5-hydroxytryptophan (5-HTP), which is subsequently decarboxylated to form serotonin. Tryptophan hydroxylase is a member of the aromatic amino acid hydroxylase family, which also includes tyrosine hydroxylase and phenylalanine hydroxylase.
Structure[edit]
Tryptophan hydroxylase is a monomeric enzyme that requires a cofactor, tetrahydrobiopterin (BH4), for its activity. The enzyme also requires molecular oxygen and iron as a cofactor. The active site of tryptophan hydroxylase contains a non-heme iron that is essential for its catalytic function. The enzyme is composed of several domains, including a regulatory domain, a catalytic domain, and a tetramerization domain.
Isoforms[edit]
There are two isoforms of tryptophan hydroxylase, known as TPH1 and TPH2. TPH1 is primarily expressed in peripheral tissues such as the pineal gland and the gastrointestinal tract, while TPH2 is predominantly found in the central nervous system. The differential expression of these isoforms allows for the regulation of serotonin synthesis in different tissues.
Function[edit]
Tryptophan hydroxylase catalyzes the hydroxylation of tryptophan to form 5-hydroxytryptophan (5-HTP). This reaction is the first and rate-limiting step in the biosynthesis of serotonin. The activity of tryptophan hydroxylase is regulated by various factors, including the availability of its substrate, tryptophan, and its cofactor, tetrahydrobiopterin. Additionally, the enzyme can be regulated by phosphorylation and by feedback inhibition from serotonin.
Clinical Significance[edit]
Alterations in tryptophan hydroxylase activity have been implicated in various disorders related to serotonin imbalance. For example, reduced activity of TPH2 has been associated with depression and anxiety disorders. Genetic polymorphisms in the TPH1 and TPH2 genes have been studied in relation to their potential impact on psychiatric conditions and response to treatment.
Related Pages[edit]
| Enzymes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| Neurotransmitters | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|