Aliphatic compound
-
Aliphatic compound
-
Aliphatic compound
-
Aliphatic compound
-
Aliphatic compound
-
Aliphatic compound
-
Aliphatic compound
-
Aliphatic compound
-
Aliphatic compound
-
Aliphatic compound
-
Aliphatic compound
-
Aliphatic compound
-
Aliphatic compound
Aliphatic Compound[edit]
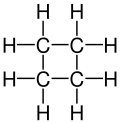
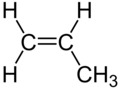
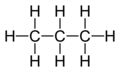
An aliphatic compound is an organic compound in which carbon atoms form open chains, as opposed to aromatic compounds that contain a benzene ring or similar structure. Aliphatic compounds can be saturated, like alkanes, or unsaturated, like alkenes and alkynes.
Classification[edit]
Aliphatic compounds are classified into three main types based on the types of bonds between carbon atoms:
- Alkanes: These are saturated hydrocarbons with single bonds between carbon atoms. They follow the general formula CnH2n+2. Examples include methane, ethane, and propane.
- Alkenes: These are unsaturated hydrocarbons containing at least one carbon-carbon double bond. They follow the general formula CnH2n. Examples include ethylene and propylene.
- Alkynes: These are unsaturated hydrocarbons with at least one carbon-carbon triple bond. They follow the general formula CnH2n-2. Examples include acetylene and propyne.
Properties[edit]
Aliphatic compounds exhibit a wide range of physical and chemical properties, which depend on the length and structure of the carbon chain and the presence of functional groups. Generally, they are less dense than water and are non-polar, making them insoluble in water but soluble in organic solvents.
- Boiling and Melting Points: The boiling and melting points of aliphatic compounds increase with the length of the carbon chain due to increased van der Waals forces.
- Reactivity: Alkanes are relatively unreactive due to the strength of the C-C and C-H bonds. Alkenes and alkynes are more reactive due to the presence of double and triple bonds, which can participate in addition reactions.
Applications[edit]
Aliphatic compounds are used in a variety of applications:
- Fuels: Many aliphatic hydrocarbons are used as fuels. For example, propane and butane are used in liquefied petroleum gas (LPG).
- Industrial Chemicals: Alkenes like ethylene and propylene are important feedstocks in the production of plastics and other chemicals.
- Solvents: Aliphatic compounds such as hexane are used as solvents in industrial and laboratory settings.
Related Pages[edit]
| Organic compounds | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
This organic compounds related article is a stub.
|
Ad. Transform your life with W8MD's Budget GLP-1 injections from $75


W8MD offers a medical weight loss program to lose weight in Philadelphia. Our physician-supervised medical weight loss provides:
- Weight loss injections in NYC (generic and brand names):
- Zepbound / Mounjaro, Wegovy / Ozempic, Saxenda
- Most insurances accepted or discounted self-pay rates. We will obtain insurance prior authorizations if needed.
- Generic GLP1 weight loss injections from $75 for the starting dose.
- Also offer prescription weight loss medications including Phentermine, Qsymia, Diethylpropion, Contrave etc.
NYC weight loss doctor appointmentsNYC weight loss doctor appointments
Start your NYC weight loss journey today at our NYC medical weight loss and Philadelphia medical weight loss clinics.
- Call 718-946-5500 to lose weight in NYC or for medical weight loss in Philadelphia 215-676-2334.
- Tags:NYC medical weight loss, Philadelphia lose weight Zepbound NYC, Budget GLP1 weight loss injections, Wegovy Philadelphia, Wegovy NYC, Philadelphia medical weight loss, Brookly weight loss and Wegovy NYC
|
WikiMD's Wellness Encyclopedia |
| Let Food Be Thy Medicine Medicine Thy Food - Hippocrates |
Medical Disclaimer: WikiMD is not a substitute for professional medical advice. The information on WikiMD is provided as an information resource only, may be incorrect, outdated or misleading, and is not to be used or relied on for any diagnostic or treatment purposes. Please consult your health care provider before making any healthcare decisions or for guidance about a specific medical condition. WikiMD expressly disclaims responsibility, and shall have no liability, for any damages, loss, injury, or liability whatsoever suffered as a result of your reliance on the information contained in this site. By visiting this site you agree to the foregoing terms and conditions, which may from time to time be changed or supplemented by WikiMD. If you do not agree to the foregoing terms and conditions, you should not enter or use this site. See full disclaimer.
Credits:Most images are courtesy of Wikimedia commons, and templates, categories Wikipedia, licensed under CC BY SA or similar.
Translate this page: - East Asian
中文,
日本,
한국어,
South Asian
हिन्दी,
தமிழ்,
తెలుగు,
Urdu,
ಕನ್ನಡ,
Southeast Asian
Indonesian,
Vietnamese,
Thai,
မြန်မာဘာသာ,
বাংলা
European
español,
Deutsch,
français,
Greek,
português do Brasil,
polski,
română,
русский,
Nederlands,
norsk,
svenska,
suomi,
Italian
Middle Eastern & African
عربى,
Turkish,
Persian,
Hebrew,
Afrikaans,
isiZulu,
Kiswahili,
Other
Bulgarian,
Hungarian,
Czech,
Swedish,
മലയാളം,
मराठी,
ਪੰਜਾਬੀ,
ગુજરાતી,
Portuguese,
Ukrainian