Aliphatic compound: Difference between revisions
From WikiMD's Wellness Encyclopedia
CSV import |
CSV import |
||
| Line 13: | Line 13: | ||
File:1-Butene.svg|Aliphatic compound | File:1-Butene.svg|Aliphatic compound | ||
</gallery> | </gallery> | ||
== Aliphatic Compound == | |||
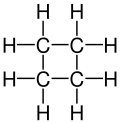
An '''aliphatic compound''' is an [[organic compound]] in which carbon atoms form open chains, as opposed to [[aromatic compounds]] that contain a [[benzene ring]] or similar structure. Aliphatic compounds can be saturated, like [[alkanes]], or unsaturated, like [[alkenes]] and [[alkynes]]. | |||
=== Classification === | |||
Aliphatic compounds are classified into three main types based on the types of bonds between carbon atoms: | |||
* '''Alkanes''': These are saturated hydrocarbons with single bonds between carbon atoms. They follow the general formula C<sub>n</sub>H<sub>2n+2</sub>. Examples include [[methane]], [[ethane]], and [[propane]]. | |||
* '''Alkenes''': These are unsaturated hydrocarbons containing at least one carbon-carbon double bond. They follow the general formula C<sub>n</sub>H<sub>2n</sub>. Examples include [[ethylene]] and [[propylene]]. | |||
* '''Alkynes''': These are unsaturated hydrocarbons with at least one carbon-carbon triple bond. They follow the general formula C<sub>n</sub>H<sub>2n-2</sub>. Examples include [[acetylene]] and [[propyne]]. | |||
=== Properties === | |||
Aliphatic compounds exhibit a wide range of physical and chemical properties, which depend on the length and structure of the carbon chain and the presence of functional groups. Generally, they are less dense than water and are non-polar, making them insoluble in water but soluble in organic solvents. | |||
* '''Boiling and Melting Points''': The boiling and melting points of aliphatic compounds increase with the length of the carbon chain due to increased [[van der Waals forces]]. | |||
* '''Reactivity''': Alkanes are relatively unreactive due to the strength of the C-C and C-H bonds. Alkenes and alkynes are more reactive due to the presence of double and triple bonds, which can participate in [[addition reactions]]. | |||
=== Applications === | |||
Aliphatic compounds are used in a variety of applications: | |||
* '''Fuels''': Many aliphatic hydrocarbons are used as fuels. For example, [[propane]] and [[butane]] are used in [[liquefied petroleum gas]] (LPG). | |||
* '''Industrial Chemicals''': Alkenes like [[ethylene]] and [[propylene]] are important feedstocks in the production of [[plastics]] and other chemicals. | |||
* '''Solvents''': Aliphatic compounds such as [[hexane]] are used as solvents in industrial and laboratory settings. | |||
== Related Pages == | |||
* [[Aromatic compound]] | |||
* [[Hydrocarbon]] | |||
* [[Functional group]] | |||
* [[Organic chemistry]] | |||
{{Organic compounds}} | |||
[[Category:Organic compounds]] | |||
Latest revision as of 00:35, 19 February 2025
-
Aliphatic compound
-
Aliphatic compound
-
Aliphatic compound
-
Aliphatic compound
-
Aliphatic compound
-
Aliphatic compound
-
Aliphatic compound
-
Aliphatic compound
-
Aliphatic compound
-
Aliphatic compound
-
Aliphatic compound
-
Aliphatic compound
Aliphatic Compound[edit]
An aliphatic compound is an organic compound in which carbon atoms form open chains, as opposed to aromatic compounds that contain a benzene ring or similar structure. Aliphatic compounds can be saturated, like alkanes, or unsaturated, like alkenes and alkynes.
Classification[edit]
Aliphatic compounds are classified into three main types based on the types of bonds between carbon atoms:
- Alkanes: These are saturated hydrocarbons with single bonds between carbon atoms. They follow the general formula CnH2n+2. Examples include methane, ethane, and propane.
- Alkenes: These are unsaturated hydrocarbons containing at least one carbon-carbon double bond. They follow the general formula CnH2n. Examples include ethylene and propylene.
- Alkynes: These are unsaturated hydrocarbons with at least one carbon-carbon triple bond. They follow the general formula CnH2n-2. Examples include acetylene and propyne.
Properties[edit]
Aliphatic compounds exhibit a wide range of physical and chemical properties, which depend on the length and structure of the carbon chain and the presence of functional groups. Generally, they are less dense than water and are non-polar, making them insoluble in water but soluble in organic solvents.
- Boiling and Melting Points: The boiling and melting points of aliphatic compounds increase with the length of the carbon chain due to increased van der Waals forces.
- Reactivity: Alkanes are relatively unreactive due to the strength of the C-C and C-H bonds. Alkenes and alkynes are more reactive due to the presence of double and triple bonds, which can participate in addition reactions.
Applications[edit]
Aliphatic compounds are used in a variety of applications:
- Fuels: Many aliphatic hydrocarbons are used as fuels. For example, propane and butane are used in liquefied petroleum gas (LPG).
- Industrial Chemicals: Alkenes like ethylene and propylene are important feedstocks in the production of plastics and other chemicals.
- Solvents: Aliphatic compounds such as hexane are used as solvents in industrial and laboratory settings.
Related Pages[edit]
| Organic compounds | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
This organic compounds related article is a stub.
|