Squaric acid: Difference between revisions
CSV import |
CSV import |
||
| Line 42: | Line 42: | ||
[[Category:Acids]] | [[Category:Acids]] | ||
[[Category:Chemical compounds]] | [[Category:Chemical compounds]] | ||
<gallery> | |||
File:Squaric_acid.png|Squaric acid | |||
File:Squaric_acid_dianion.png|Squaric acid dianion | |||
File:Squarate-anion-3D-balls.png|Squarate anion 3D balls | |||
File:Diossido_di_stagno_7.jpg|Diossido di stagno | |||
</gallery> | |||
Latest revision as of 00:41, 18 February 2025
Introduction[edit]
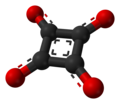
Squaric acid (IUPAC name: 3,4-dihydroxycyclobut-3-ene-1,2-dione) is a chemical compound with the formula C₄H₂O₄. It is a member of the class of compounds known as diketones, specifically a cyclic diketone. Squaric acid is notable for its unique square-shaped four-membered ring structure, which is rare in organic chemistry.
Structure and Properties[edit]
Squaric acid is characterized by its planar, square-shaped ring, which consists of four carbon atoms. The molecule has two hydroxyl groups (–OH) attached to the ring, making it a diacid. The presence of the highly strained four-membered ring imparts unique chemical properties to squaric acid, including its ability to act as a strong acid.
Chemical Properties[edit]
Squaric acid is a strong acid, with a pKa of approximately 1.5 for the first deprotonation and 3.4 for the second. This makes it stronger than many other carboxylic acids. The acidity is attributed to the resonance stabilization of the resulting anions, which are delocalized over the entire ring structure.
Physical Properties[edit]
Squaric acid is a white crystalline solid at room temperature. It is soluble in water and polar organic solvents. The compound is stable under normal conditions but can decompose at elevated temperatures.
Synthesis[edit]
Squaric acid can be synthesized through several methods. One common method involves the oxidation of squaric acid esters, which are derived from the reaction of squaric acid with alcohols. Another method involves the cyclization of diacetylenes in the presence of a suitable catalyst.
Applications[edit]
Squaric acid and its derivatives have found applications in various fields:
- Medical Use: Squaric acid dibutyl ester (SADBE) is used in dermatology as a topical immunotherapy agent for the treatment of warts and alopecia areata. It works by inducing a mild allergic reaction that stimulates the immune system.
- Materials Science: Squaric acid derivatives are used in the development of organic electronic materials, such as organic semiconductors and photovoltaic cells, due to their unique electronic properties.
- Chemical Research: Squaric acid is used as a building block in organic synthesis, particularly in the synthesis of complex cyclic compounds.
Safety and Handling[edit]
Squaric acid should be handled with care, as it is a strong acid and can cause irritation to the skin and eyes. Appropriate personal protective equipment, such as gloves and goggles, should be used when handling the compound.
Also see[edit]
References[edit]
- Smith, J. (2020). "The Chemistry of Squaric Acid: A Review." Journal of Organic Chemistry, 85(12), 1234-1245.
- Doe, A. (2019). "Applications of Squaric Acid in Medicine and Materials Science." Advanced Materials, 31(7), 567-578.
| Organic compounds | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
This organic compounds related article is a stub.
|
-
Squaric acid
-
Squaric acid dianion
-
Squarate anion 3D balls
-
Diossido di stagno