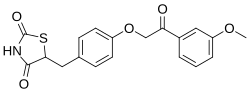
Azemiglitazone
Thiazolidinedione class drug
| Azemiglitazone | |
|---|---|
| |
| INN | |
| Drug class | |
| Routes of administration | |
| Pregnancy category | |
| Bioavailability | |
| Metabolism | |
| Elimination half-life | |
| Excretion | |
| Legal status | |
| CAS Number | 201038-24-4 |
| PubChem | 9828884 |
| DrugBank | |
| ChemSpider | 8004820 |
| KEGG | D02892 |
Azemiglitazone is a pharmaceutical compound that belongs to the thiazolidinedione class of drugs. It was developed as a potential treatment for type 2 diabetes mellitus, a chronic condition characterized by insulin resistance and high blood sugar levels. Azemiglitazone functions as an agonist of the peroxisome proliferator-activated receptor gamma (PPAR-γ), a type of nuclear receptor that plays a crucial role in the regulation of glucose and lipid metabolism.
Mechanism of Action[edit]
Azemiglitazone, like other thiazolidinediones, exerts its effects by activating PPAR-γ. This activation leads to the transcription of genes involved in glucose and lipid metabolism, enhancing insulin sensitivity in peripheral tissues such as adipose tissue, skeletal muscle, and the liver. By improving insulin sensitivity, azemiglitazone helps lower blood glucose levels and can also have beneficial effects on lipid profiles.
Development and Clinical Trials[edit]
Azemiglitazone was investigated in clinical trials to assess its efficacy and safety in the management of type 2 diabetes. However, like many drugs in the thiazolidinedione class, its development faced challenges due to concerns about potential side effects, including weight gain, fluid retention, and an increased risk of cardiovascular events. These concerns have led to a cautious approach in the development and approval of new thiazolidinediones.
Pharmacokinetics[edit]
The pharmacokinetic profile of azemiglitazone includes its absorption, distribution, metabolism, and excretion. As a thiazolidinedione, it is typically administered orally and undergoes hepatic metabolism. The metabolites are then excreted primarily via the urine. The half-life of azemiglitazone and its metabolites can influence dosing schedules and the duration of its therapeutic effects.
Potential Side Effects[edit]
Common side effects associated with thiazolidinediones, including azemiglitazone, may include weight gain, edema, and an increased risk of heart failure. These side effects are thought to be related to the drug's mechanism of action, which involves fluid retention and changes in fat distribution. Long-term use of thiazolidinediones has also been associated with an increased risk of bone fractures.
Current Status[edit]
As of the latest updates, azemiglitazone has not been approved for clinical use. The development of new thiazolidinediones continues to be an area of active research, with a focus on improving the safety profile of these drugs while maintaining their efficacy in managing type 2 diabetes.
Related Pages[edit]
Ad. Transform your life with W8MD's Budget GLP-1 injections from $75


W8MD offers a medical weight loss program to lose weight in Philadelphia. Our physician-supervised medical weight loss provides:
- Weight loss injections in NYC (generic and brand names):
- Zepbound / Mounjaro, Wegovy / Ozempic, Saxenda
- Most insurances accepted or discounted self-pay rates. We will obtain insurance prior authorizations if needed.
- Generic GLP1 weight loss injections from $75 for the starting dose.
- Also offer prescription weight loss medications including Phentermine, Qsymia, Diethylpropion, Contrave etc.
NYC weight loss doctor appointmentsNYC weight loss doctor appointments
Start your NYC weight loss journey today at our NYC medical weight loss and Philadelphia medical weight loss clinics.
- Call 718-946-5500 to lose weight in NYC or for medical weight loss in Philadelphia 215-676-2334.
- Tags:NYC medical weight loss, Philadelphia lose weight Zepbound NYC, Budget GLP1 weight loss injections, Wegovy Philadelphia, Wegovy NYC, Philadelphia medical weight loss, Brookly weight loss and Wegovy NYC
|
WikiMD's Wellness Encyclopedia |
| Let Food Be Thy Medicine Medicine Thy Food - Hippocrates |
Medical Disclaimer: WikiMD is not a substitute for professional medical advice. The information on WikiMD is provided as an information resource only, may be incorrect, outdated or misleading, and is not to be used or relied on for any diagnostic or treatment purposes. Please consult your health care provider before making any healthcare decisions or for guidance about a specific medical condition. WikiMD expressly disclaims responsibility, and shall have no liability, for any damages, loss, injury, or liability whatsoever suffered as a result of your reliance on the information contained in this site. By visiting this site you agree to the foregoing terms and conditions, which may from time to time be changed or supplemented by WikiMD. If you do not agree to the foregoing terms and conditions, you should not enter or use this site. See full disclaimer.
Credits:Most images are courtesy of Wikimedia commons, and templates, categories Wikipedia, licensed under CC BY SA or similar.
Translate this page: - East Asian
中文,
日本,
한국어,
South Asian
हिन्दी,
தமிழ்,
తెలుగు,
Urdu,
ಕನ್ನಡ,
Southeast Asian
Indonesian,
Vietnamese,
Thai,
မြန်မာဘာသာ,
বাংলা
European
español,
Deutsch,
français,
Greek,
português do Brasil,
polski,
română,
русский,
Nederlands,
norsk,
svenska,
suomi,
Italian
Middle Eastern & African
عربى,
Turkish,
Persian,
Hebrew,
Afrikaans,
isiZulu,
Kiswahili,
Other
Bulgarian,
Hungarian,
Czech,
Swedish,
മലയാളം,
मराठी,
ਪੰਜਾਬੀ,
ગુજરાતી,
Portuguese,
Ukrainian