Ammonium bromide: Difference between revisions
CSV import |
CSV import |
||
| Line 5: | Line 5: | ||
File:Levonorgestrel.svg|Structural formula of levonorgestrel | File:Levonorgestrel.svg|Structural formula of levonorgestrel | ||
</gallery> | </gallery> | ||
== Ammonium Bromide == | |||
'''Ammonium bromide''' is an inorganic compound with the chemical formula NH_Br. It is the ammonium salt of hydrobromic acid and appears as a white crystalline solid that is highly soluble in water. | |||
== Properties == | |||
Ammonium bromide is a white, crystalline powder that is highly soluble in water. It has a melting point of 452 °C and decomposes upon heating to release ammonia and hydrogen bromide gases. The compound is hygroscopic, meaning it can absorb moisture from the air, which can lead to clumping if not stored properly. | |||
== Chemical Structure == | |||
The chemical structure of ammonium bromide consists of the ammonium ion (NH__) and the bromide ion (Br_). The ammonium ion is a positively charged polyatomic ion, while the bromide ion is a negatively charged halide ion. Together, they form an ionic compound. | |||
== Uses == | |||
Ammonium bromide is used in various applications, including: | |||
* '''Photography''': It is used in the preparation of photographic films and papers. Ammonium bromide is involved in the formation of silver bromide, which is a light-sensitive compound used in photographic emulsions. | |||
* '''Pharmaceuticals''': It is used in the synthesis of certain pharmaceuticals and as a sedative in veterinary medicine. | |||
* '''Chemical Synthesis''': Ammonium bromide is used as a source of bromide ions in chemical reactions and as a catalyst in some organic synthesis processes. | |||
* '''Fire Retardants''': It is used in the formulation of fire retardant materials. | |||
== Safety == | |||
Ammonium bromide should be handled with care, as it can release toxic gases when heated. It can cause irritation to the skin, eyes, and respiratory tract. Proper protective equipment, such as gloves and goggles, should be used when handling the compound. | |||
== Related Compounds == | |||
Ammonium bromide is related to other ammonium halides, such as [[ammonium chloride]] (NH_Cl) and [[ammonium iodide]] (NH_I). These compounds share similar properties and uses, particularly in their roles as sources of halide ions in various chemical processes. | |||
== Related Pages == | |||
* [[Ammonium chloride]] | |||
* [[Ammonium iodide]] | |||
* [[Hydrobromic acid]] | |||
* [[Silver bromide]] | |||
* [[Halide]] | |||
{{Chemistry}} | |||
{{Inorganic compounds}} | |||
[[Category:Ammonium compounds]] | |||
[[Category:Bromides]] | |||
[[Category:Photographic chemicals]] | |||
Latest revision as of 00:42, 19 February 2025
Ammonium_bromide[edit]
-
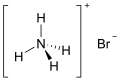
Structural formula of ammonium bromide
-
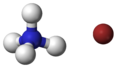
3D ball model of ammonium bromide
-
Structural formula of levonorgestrel
Ammonium Bromide[edit]
Ammonium bromide is an inorganic compound with the chemical formula NH_Br. It is the ammonium salt of hydrobromic acid and appears as a white crystalline solid that is highly soluble in water.
Properties[edit]
Ammonium bromide is a white, crystalline powder that is highly soluble in water. It has a melting point of 452 °C and decomposes upon heating to release ammonia and hydrogen bromide gases. The compound is hygroscopic, meaning it can absorb moisture from the air, which can lead to clumping if not stored properly.
Chemical Structure[edit]
The chemical structure of ammonium bromide consists of the ammonium ion (NH__) and the bromide ion (Br_). The ammonium ion is a positively charged polyatomic ion, while the bromide ion is a negatively charged halide ion. Together, they form an ionic compound.
Uses[edit]
Ammonium bromide is used in various applications, including:
- Photography: It is used in the preparation of photographic films and papers. Ammonium bromide is involved in the formation of silver bromide, which is a light-sensitive compound used in photographic emulsions.
- Pharmaceuticals: It is used in the synthesis of certain pharmaceuticals and as a sedative in veterinary medicine.
- Chemical Synthesis: Ammonium bromide is used as a source of bromide ions in chemical reactions and as a catalyst in some organic synthesis processes.
- Fire Retardants: It is used in the formulation of fire retardant materials.
Safety[edit]
Ammonium bromide should be handled with care, as it can release toxic gases when heated. It can cause irritation to the skin, eyes, and respiratory tract. Proper protective equipment, such as gloves and goggles, should be used when handling the compound.
Related Compounds[edit]
Ammonium bromide is related to other ammonium halides, such as ammonium chloride (NH_Cl) and ammonium iodide (NH_I). These compounds share similar properties and uses, particularly in their roles as sources of halide ions in various chemical processes.
Related Pages[edit]
| Branches of chemistry | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| Inorganic compounds | ||||||||
|---|---|---|---|---|---|---|---|---|
This inorganic compounds related article is a stub.
|