Golodirsen
What is Golodirsen?[edit]
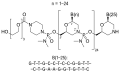
- Golodirsen (VYONDYS 53) is an antisense oligonucleotide used for the treatment of Duchenne muscular dystrophy (DMD).
What are the uses of this medicine?[edit]
- This medicine is used for the treatment of Duchenne muscular dystrophy (DMD) in patients who have a confirmed mutation of the DMD gene that is amenable to exon 53 skipping.
How does this medicine work?[edit]
- Golodirsen is designed to bind to exon 53 of dystrophin pre-mRNA resulting in exclusion of this exon during mRNA processing in patients with genetic mutations that are amenable to exon 53 skipping.
- Exon 53 skipping is intended to allow for production of an internally truncated dystrophin protein in patients with genetic mutations that are amenable to exon 53 skipping.
Who Should Not Use this medicine ?[edit]
- This medicine have no usage limitattions.
Is this medicine FDA approved?[edit]
- It was approved for use in the United States in 2019.
How should this medicine be used?[edit]
- Serum cystatin C, urine dipstick, and urine protein-to-creatinine ratio should be measured before starting VYONDYS 53.
- Consider measurement of glomerular filtration rate prior to initiation of VYONDYS 53.
- Monitoring for kidney toxicity during treatment is recommended.
- Obtain the urine samples prior to infusion of VYONDYS 53 or at least 48 hours after the most recent infusion.
Recommended Dosage
- The recommended dosage of VYONDYS 53 is 30 milligrams per kilogram administered once weekly as a 35 to 60-minute intravenous infusion via an in-line 0.2 micron filter.
- Dilution required prior to administration.
Administration
- Application of a topical anesthetic cream to the infusion site prior to administration of VYONDYS 53 may be considered.
- VYONDYS 53 is administered via intravenous infusion. Flush the intravenous access line with 0.9% Sodium Chloride Injection, USP, prior to and after infusion.
- Infuse the diluted VYONDYS 53 over 35 to 60 minutes via an in-line 0.2 micron filter. Do not mix other medications with VYONDYS 53 or infuse other medications concomitantly via the same intravenous access line with VYONDYS 53.
- If a hypersensitivity reaction occurs, consider slowing the infusion or interrupting the VYONDYS 53 therapy.
What are the dosage forms and brand names of this medicine?[edit]
This medicine is available in fallowing doasage form:
- Injection: 100 mg/2 mL (50 mg/mL) in a single-dose vial.
This medicine is available in fallowing brand namesː
- VYONDYS 53
What side effects can this medication cause?[edit]
The most common side effects of this medicine include:
- headache
- pyrexia
- fall
- abdominal pain
- nasopharyngitis
- cough
- vomiting
- nausea
What special precautions should I follow?[edit]
- Hypersensitivity reactions, including rash, pyrexia, pruritus, urticaria, dermatitis, and skin exfoliation have occurred in patients who were treated with VYONDYS 53. If a hypersensitivity reaction occurs, institute appropriate medical treatment and consider slowing the infusion or interrupting the VYONDYS 53 therapy.
- Based on animal data, may cause kidney toxicity. Kidney function should be monitored; creatinine may not be a reliable measure of renal function in DMD patients.
What to do in case of emergency/overdose?[edit]
- In case of overdose, call the poison control helpline of your country. In the United States, call 1-800-222-1222.
- Overdose related information is also available online at poisonhelp.org/help.
- In the event that the victim has collapsed, had a seizure, has trouble breathing, or can't be awakened, immediately call emergency services. In the United States, call 911.
Can this medicine be used in pregnancy?[edit]
- There are no human or animal data available to assess the use of VYONDYS 53 during pregnancy.
Can this medicine be used in children?[edit]
- VYONDYS 53 is indicated for the treatment of Duchenne muscular dystrophy (DMD) in patients who have a confirmed mutation of the DMD gene that is amenable to exon 53 skipping, including pediatric patients.
What are the active and inactive ingredients in this medicine?[edit]
Active Ingredient:
- golodirsen
Inactive Ingredients:
- sodium chloride
- potassium chloride
- potassium phosphate, monobasic
- sodium phosphate, dibasic, anhydrous
- sodium hydroxide
- hydrochloric acid
- water
Who manufactures and distributes this medicine?[edit]
- Mfg for: Sarepta Therapeutics,
Inc., Cambridge, MA 02142
What should I know about storage and disposal of this medication?[edit]
- Store VYONDYS 53 at 2°C to 8°C (36°F to 46°F).
- Do not freeze.
- Store in original carton until ready for use to protect from light.
| Other drugs for disorders of the musculo-skeletal system (M09) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
-
Golodirsen
Ad. Transform your life with W8MD's Budget GLP-1 injections from $75


W8MD offers a medical weight loss program to lose weight in Philadelphia. Our physician-supervised medical weight loss provides:
- Weight loss injections in NYC (generic and brand names):
- Zepbound / Mounjaro, Wegovy / Ozempic, Saxenda
- Most insurances accepted or discounted self-pay rates. We will obtain insurance prior authorizations if needed.
- Generic GLP1 weight loss injections from $75 for the starting dose.
- Also offer prescription weight loss medications including Phentermine, Qsymia, Diethylpropion, Contrave etc.
NYC weight loss doctor appointmentsNYC weight loss doctor appointments
Start your NYC weight loss journey today at our NYC medical weight loss and Philadelphia medical weight loss clinics.
- Call 718-946-5500 to lose weight in NYC or for medical weight loss in Philadelphia 215-676-2334.
- Tags:NYC medical weight loss, Philadelphia lose weight Zepbound NYC, Budget GLP1 weight loss injections, Wegovy Philadelphia, Wegovy NYC, Philadelphia medical weight loss, Brookly weight loss and Wegovy NYC
|
WikiMD's Wellness Encyclopedia |
| Let Food Be Thy Medicine Medicine Thy Food - Hippocrates |
Medical Disclaimer: WikiMD is not a substitute for professional medical advice. The information on WikiMD is provided as an information resource only, may be incorrect, outdated or misleading, and is not to be used or relied on for any diagnostic or treatment purposes. Please consult your health care provider before making any healthcare decisions or for guidance about a specific medical condition. WikiMD expressly disclaims responsibility, and shall have no liability, for any damages, loss, injury, or liability whatsoever suffered as a result of your reliance on the information contained in this site. By visiting this site you agree to the foregoing terms and conditions, which may from time to time be changed or supplemented by WikiMD. If you do not agree to the foregoing terms and conditions, you should not enter or use this site. See full disclaimer.
Credits:Most images are courtesy of Wikimedia commons, and templates, categories Wikipedia, licensed under CC BY SA or similar.
Translate this page: - East Asian
中文,
日本,
한국어,
South Asian
हिन्दी,
தமிழ்,
తెలుగు,
Urdu,
ಕನ್ನಡ,
Southeast Asian
Indonesian,
Vietnamese,
Thai,
မြန်မာဘာသာ,
বাংলা
European
español,
Deutsch,
français,
Greek,
português do Brasil,
polski,
română,
русский,
Nederlands,
norsk,
svenska,
suomi,
Italian
Middle Eastern & African
عربى,
Turkish,
Persian,
Hebrew,
Afrikaans,
isiZulu,
Kiswahili,
Other
Bulgarian,
Hungarian,
Czech,
Swedish,
മലയാളം,
मराठी,
ਪੰਜਾਬੀ,
ગુજરાતી,
Portuguese,
Ukrainian