Bioconjugation: Difference between revisions
CSV import |
CSV import Tags: mobile edit mobile web edit |
||
| Line 52: | Line 52: | ||
[[Category:Chemistry]] | [[Category:Chemistry]] | ||
[[Category:Bioconjugation]] | [[Category:Bioconjugation]] | ||
<gallery> | |||
File:Ibritumomab_tiuxetan_structure_v01.svg|Ibritumomab tiuxetan structure | |||
File:Bioconjugation_strategies_for_lysine_residues.tif|Bioconjugation strategies for lysine residues | |||
File:Bioconjugation_strategies_for_cysteine_residues.tif|Bioconjugation strategies for cysteine residues | |||
File:Bioconjugation_strategies_for_tyrosine_residues.tif|Bioconjugation strategies for tyrosine residues | |||
File:Figure_4._Bioconjugation_strategies_for_N-terminus.tif|Bioconjugation strategies for N-terminus | |||
File:Figure_5._Bioconjugation_strategies_for_C-terminus.tif|Bioconjugation strategies for C-terminus | |||
File:Figure_6._Bioconjugation_strategies_for_targeting_ketones_and_aldehydes.jpg|Bioconjugation strategies for targeting ketones and aldehydes | |||
File:Figure_7._Nucleophilic_catalysis_of_oxime_ligation.jpg|Nucleophilic catalysis of oxime ligation | |||
</gallery> | |||
Latest revision as of 00:59, 27 February 2025
Bioconjugation
Bioconjugation is a chemical strategy used to form a stable covalent link between two molecules, one of which is typically a biomolecule. This process is crucial in the development of various biotechnological and therapeutic applications, including the creation of antibody-drug conjugates, biosensors, and imaging agents.
Overview[edit]
Bioconjugation involves the attachment of a biomolecule, such as a protein, nucleic acid, or carbohydrate, to another molecule, which can be a small synthetic compound, another biomolecule, or a nanoparticle. The goal is to combine the unique properties of each component to create a new entity with enhanced or novel functionalities.
Techniques[edit]
Several techniques are employed in bioconjugation, each with its own advantages and limitations. Some of the most common methods include:
Amide Bond Formation[edit]
Amide bond formation is one of the most widely used bioconjugation techniques. It involves the reaction between a carboxylic acid group and an amine group to form an amide bond. This method is often used to attach peptides to other molecules.
Click Chemistry[edit]
Click chemistry is a class of bioconjugation reactions that are highly efficient, selective, and occur under mild conditions. The most popular click chemistry reaction is the copper-catalyzed azide-alkyne cycloaddition (CuAAC), which forms a stable triazole linkage.
Thiol-Maleimide Reaction[edit]
The thiol-maleimide reaction is a specific and efficient method for conjugating molecules containing thiol groups, such as cysteine residues in proteins, to maleimide-functionalized compounds.
Enzymatic Conjugation[edit]
Enzymatic methods utilize enzymes to catalyze the formation of covalent bonds between biomolecules. These methods are often highly specific and can occur under physiological conditions.
Applications[edit]
Bioconjugation has a wide range of applications in various fields:
Therapeutics[edit]
In therapeutics, bioconjugation is used to create antibody-drug conjugates (ADCs), which are designed to deliver cytotoxic drugs specifically to cancer cells, minimizing damage to healthy tissues.
Diagnostics[edit]
Bioconjugation is employed in the development of biosensors and diagnostic assays, where biomolecules are conjugated to reporter molecules to detect specific analytes.
Imaging[edit]
In medical imaging, bioconjugation is used to attach imaging agents to targeting molecules, allowing for the visualization of specific tissues or disease sites.
Challenges[edit]
Despite its many applications, bioconjugation faces several challenges, including:
- Specificity: Achieving selective conjugation without affecting the biological activity of the biomolecule.
- Stability: Ensuring the stability of the conjugate under physiological conditions.
- Scalability: Developing methods that are scalable for industrial applications.
Also see[edit]
| Biotechnology | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| Branches of chemistry | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
-
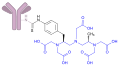
Ibritumomab tiuxetan structure
-
Bioconjugation strategies for lysine residues
-
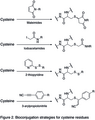
Bioconjugation strategies for cysteine residues
-
Bioconjugation strategies for tyrosine residues
-
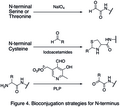
Bioconjugation strategies for N-terminus
-
Bioconjugation strategies for C-terminus
-
Bioconjugation strategies for targeting ketones and aldehydes
-
Nucleophilic catalysis of oxime ligation