4-Chlorokynurenine: Difference between revisions
CSV import |
CSV import |
||
| Line 1: | Line 1: | ||
{{Short description|A chemical compound used in research}} | |||
{{DISPLAYTITLE:4-Chlorokynurenine}} | |||
'''4-Chlorokynurenine''' is a chemical compound that | '''4-Chlorokynurenine''' is a chemical compound that has been studied for its potential effects on the [[nervous system]]. It is a derivative of [[kynurenine]], an intermediate in the [[tryptophan]] metabolism pathway. 4-Chlorokynurenine is of interest in [[neuroscience]] research due to its role as a prodrug for 7-chlorokynurenic acid, a potent antagonist of the [[NMDA receptor]]. | ||
==Chemical Structure== | |||
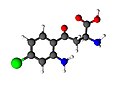
4-Chlorokynurenine is | 4-Chlorokynurenine is structurally similar to kynurenine, with the addition of a chlorine atom at the 4-position of the aromatic ring. This modification alters its pharmacological properties, making it a subject of interest in the study of [[neurotransmitter]] systems. | ||
==Mechanism of Action== | |||
4-Chlorokynurenine is | 4-Chlorokynurenine is metabolized in the body to produce 7-chlorokynurenic acid, which acts as an antagonist at the [[glycine]] site of the [[NMDA receptor]]. The NMDA receptor is a type of [[glutamate receptor]] that plays a crucial role in [[synaptic plasticity]] and [[memory]] function. By inhibiting this receptor, 4-chlorokynurenine may modulate [[excitatory neurotransmission]] and has been investigated for its potential therapeutic effects in [[neurological disorders]]. | ||
== | ==Research and Applications== | ||
Research into 4-chlorokynurenine has focused on its potential use in treating conditions such as [[depression]], [[schizophrenia]], and [[neurodegenerative diseases]]. Its ability to cross the [[blood-brain barrier]] and be converted into an active NMDA receptor antagonist makes it a promising candidate for further study. | |||
== | ==Safety and Toxicology== | ||
As with many experimental compounds, the safety profile of 4-chlorokynurenine is not fully established. Studies are ongoing to determine its potential side effects and toxicological properties in both animal models and human subjects. | |||
==Related pages== | |||
== Related | |||
* [[Kynurenine pathway]] | * [[Kynurenine pathway]] | ||
* [[NMDA receptor]] | * [[NMDA receptor]] | ||
* [[Neurotransmitter]] | |||
* [[Glutamate receptor]] | * [[Glutamate receptor]] | ||
== Gallery == | ==Gallery== | ||
<gallery> | <gallery> | ||
File:4-Chlorokynurenine.svg|Chemical structure of 4-Chlorokynurenine | File:4-Chlorokynurenine.svg|Chemical structure of 4-Chlorokynurenine | ||
| Line 35: | Line 29: | ||
</gallery> | </gallery> | ||
[[Category: | [[Category:Chemical compounds]] | ||
[[Category: | [[Category:Neuroscience]] | ||
[[Category: | [[Category:NMDA receptor antagonists]] | ||
Revision as of 20:05, 11 February 2025
A chemical compound used in research
4-Chlorokynurenine is a chemical compound that has been studied for its potential effects on the nervous system. It is a derivative of kynurenine, an intermediate in the tryptophan metabolism pathway. 4-Chlorokynurenine is of interest in neuroscience research due to its role as a prodrug for 7-chlorokynurenic acid, a potent antagonist of the NMDA receptor.
Chemical Structure
4-Chlorokynurenine is structurally similar to kynurenine, with the addition of a chlorine atom at the 4-position of the aromatic ring. This modification alters its pharmacological properties, making it a subject of interest in the study of neurotransmitter systems.
Mechanism of Action
4-Chlorokynurenine is metabolized in the body to produce 7-chlorokynurenic acid, which acts as an antagonist at the glycine site of the NMDA receptor. The NMDA receptor is a type of glutamate receptor that plays a crucial role in synaptic plasticity and memory function. By inhibiting this receptor, 4-chlorokynurenine may modulate excitatory neurotransmission and has been investigated for its potential therapeutic effects in neurological disorders.
Research and Applications
Research into 4-chlorokynurenine has focused on its potential use in treating conditions such as depression, schizophrenia, and neurodegenerative diseases. Its ability to cross the blood-brain barrier and be converted into an active NMDA receptor antagonist makes it a promising candidate for further study.
Safety and Toxicology
As with many experimental compounds, the safety profile of 4-chlorokynurenine is not fully established. Studies are ongoing to determine its potential side effects and toxicological properties in both animal models and human subjects.
Related pages
Gallery
-
Chemical structure of 4-Chlorokynurenine
-
Ball-and-stick model of 4-Chlorokynurenine
-
Illustration of an activated NMDA receptor