Combustion




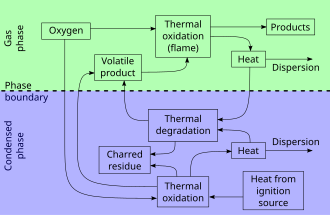
Combustion is a high-temperature exothermic chemical reaction between a fuel and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combustion in a fire produces a flame, and the heat produced can make combustion self-sustaining. Combustion is often a complex sequence of elementary radical reactions. Solid or liquid fuels undergo rapid pyrolysis or vaporization at the surface and release large quantities of volatile gases.
Combustion is an essential process in many aspects of modern life, from the operation of internal combustion engines in vehicles to the generation of electricity in power plants and the basic process of cooking and heating in homes. Understanding the principles of combustion is crucial for the design and operation of a wide range of equipment and is a central field in the study of chemical engineering, mechanical engineering, and fire safety engineering.
Types of Combustion[edit]
Combustion can be classified into several types based on the oxygen supply and the combustion characteristics:
- Complete combustion occurs when there is enough oxygen to allow the fuel to react completely to produce carbon dioxide and water, along with a maximum amount of heat. This type of combustion is desired in gasoline engines and furnaces where a clean and efficient burn is required.
- Incomplete combustion happens when there is insufficient oxygen for the fuel to react completely. It produces carbon monoxide, soot, or other partially oxidized products alongside carbon dioxide and water. Incomplete combustion is less efficient and can be hazardous due to the production of carbon monoxide.
- Spontaneous combustion occurs when a material self-ignites without the application of external heat. This can happen due to chemical reactions, biological processes, or the accumulation of heat due to poor heat dissipation.
- Rapid combustion is a form of combustion in which large amounts of heat and light are produced in a short period, often accompanied by a flame. This is the type of combustion observed in most fires.
- Explosive combustion or detonation occurs when the combustion front moves through the unburned fuel at a speed greater than the speed of sound. Explosives and certain types of engines, like the pulse jet engine, utilize this form of combustion.
Chemistry of Combustion[edit]
The basic chemical equation for combustion is typically represented as: \[ \text{Fuel} + \text{O}_2 \rightarrow \text{CO}_2 + \text{H}_2\text{O} + \text{heat} \] However, the actual process is much more complex and involves a series of intermediate reactions and the formation of various intermediates.
The key to the combustion process is the breaking and forming of chemical bonds. Energy is required to break bonds in the fuel and oxygen molecules, and energy is released when new bonds form in the combustion products. The overall process releases energy because the energy required to break the bonds is less than the energy released when new bonds are formed.
Environmental Impact[edit]
Combustion processes are a major source of air pollution. They produce carbon dioxide, a greenhouse gas; nitrogen oxides, which contribute to smog and acid rain; and particulate matter, which can affect respiratory health. Reducing the environmental impact of combustion is a significant focus of environmental engineering and sustainable development.
See Also[edit]
Ad. Transform your life with W8MD's Budget GLP-1 injections from $75


W8MD offers a medical weight loss program to lose weight in Philadelphia. Our physician-supervised medical weight loss provides:
- Weight loss injections in NYC (generic and brand names):
- Zepbound / Mounjaro, Wegovy / Ozempic, Saxenda
- Most insurances accepted or discounted self-pay rates. We will obtain insurance prior authorizations if needed.
- Generic GLP1 weight loss injections from $75 for the starting dose.
- Also offer prescription weight loss medications including Phentermine, Qsymia, Diethylpropion, Contrave etc.
NYC weight loss doctor appointmentsNYC weight loss doctor appointments
Start your NYC weight loss journey today at our NYC medical weight loss and Philadelphia medical weight loss clinics.
- Call 718-946-5500 to lose weight in NYC or for medical weight loss in Philadelphia 215-676-2334.
- Tags:NYC medical weight loss, Philadelphia lose weight Zepbound NYC, Budget GLP1 weight loss injections, Wegovy Philadelphia, Wegovy NYC, Philadelphia medical weight loss, Brookly weight loss and Wegovy NYC
|
WikiMD's Wellness Encyclopedia |
| Let Food Be Thy Medicine Medicine Thy Food - Hippocrates |
Medical Disclaimer: WikiMD is not a substitute for professional medical advice. The information on WikiMD is provided as an information resource only, may be incorrect, outdated or misleading, and is not to be used or relied on for any diagnostic or treatment purposes. Please consult your health care provider before making any healthcare decisions or for guidance about a specific medical condition. WikiMD expressly disclaims responsibility, and shall have no liability, for any damages, loss, injury, or liability whatsoever suffered as a result of your reliance on the information contained in this site. By visiting this site you agree to the foregoing terms and conditions, which may from time to time be changed or supplemented by WikiMD. If you do not agree to the foregoing terms and conditions, you should not enter or use this site. See full disclaimer.
Credits:Most images are courtesy of Wikimedia commons, and templates, categories Wikipedia, licensed under CC BY SA or similar.
Translate this page: - East Asian
中文,
日本,
한국어,
South Asian
हिन्दी,
தமிழ்,
తెలుగు,
Urdu,
ಕನ್ನಡ,
Southeast Asian
Indonesian,
Vietnamese,
Thai,
မြန်မာဘာသာ,
বাংলা
European
español,
Deutsch,
français,
Greek,
português do Brasil,
polski,
română,
русский,
Nederlands,
norsk,
svenska,
suomi,
Italian
Middle Eastern & African
عربى,
Turkish,
Persian,
Hebrew,
Afrikaans,
isiZulu,
Kiswahili,
Other
Bulgarian,
Hungarian,
Czech,
Swedish,
മലയാളം,
मराठी,
ਪੰਜਾਬੀ,
ગુજરાતી,
Portuguese,
Ukrainian
