Tyrosine: Difference between revisions
CSV import |
CSV import |
||
| Line 45: | Line 45: | ||
[[Category:Amino acids]] | [[Category:Amino acids]] | ||
[[Category:Biochemistry]] | [[Category:Biochemistry]] | ||
<gallery> | |||
File:Tyrosine-spin.gif|Tyrosine | |||
File:L-Tyrosin_-_L-Tyrosine.svg|L-Tyrosine structure | |||
File:Tyrosine_biosynthesis.svg|Tyrosine biosynthesis pathway | |||
File:Conversion_of_phenylalanine_and_tyrosine_to_its_biologically_important_derivatives.png|Conversion of phenylalanine and tyrosine to its biologically important derivatives | |||
File:Tyrosinedegradation2.png|Tyrosine degradation pathway | |||
File:Phe_Tyr.png|Phenylalanine and Tyrosine | |||
</gallery> | |||
Latest revision as of 10:59, 18 February 2025
Tyrosine[edit]


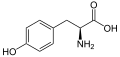
Tyrosine is an amino acid that is used in the biosynthesis of proteins. It is a non-essential amino acid with the chemical formula C_H__NO_. Tyrosine is encoded by the genetic code as the codons UAC and UAU.
Structure and Properties[edit]
Tyrosine is classified as a polar amino acid due to the presence of a hydroxyl group in its side chain. This hydroxyl group makes tyrosine more hydrophilic than other amino acids such as phenylalanine. The presence of the aromatic ring in its structure also allows tyrosine to participate in pi stacking interactions.
Biosynthesis[edit]

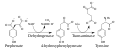
In plants and microorganisms, tyrosine is synthesized from prephenate, a product of the shikimate pathway. In animals, tyrosine is derived from phenylalanine through the action of the enzyme phenylalanine hydroxylase. This conversion is crucial for the metabolism of phenylalanine and the production of tyrosine.
Biological Role[edit]
Tyrosine is a precursor to several important neurotransmitters and hormones, including dopamine, norepinephrine, and epinephrine. It is also involved in the synthesis of melanin, the pigment responsible for the color of skin and hair.
Metabolism[edit]

Tyrosine is metabolized in the liver by the enzyme tyrosine aminotransferase, which converts it into p-hydroxyphenylpyruvate. This compound is further metabolized into homogentisate, which is eventually broken down into fumarate and acetoacetate, intermediates in the citric acid cycle.
Degradation[edit]

The degradation of tyrosine involves several steps, ultimately leading to the production of energy and the generation of metabolic intermediates. Disorders in tyrosine metabolism can lead to conditions such as tyrosinemia and alcaptonuria.
Related Pages[edit]
References[edit]
<references group="" responsive="1"></references>
-
Tyrosine
-
L-Tyrosine structure
-
Tyrosine biosynthesis pathway
-
Conversion of phenylalanine and tyrosine to its biologically important derivatives
-
Tyrosine degradation pathway
-
Phenylalanine and Tyrosine