Malic acid: Difference between revisions
CSV import Tags: mobile edit mobile web edit |
CSV import |
||
| Line 24: | Line 24: | ||
{{stub}} | {{stub}} | ||
{{dictionary-stub1}} | {{dictionary-stub1}} | ||
<gallery> | |||
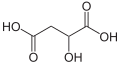
File:Äpfelsäure3.svg|Malic acid structure | |||
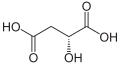
File:L-Äpfelsäure.svg|L-Malic acid structure | |||
File:D-Äpfelsäure.svg|D-Malic acid structure | |||
</gallery> | |||
Latest revision as of 01:58, 18 February 2025
Malic acid is a organic compound that is found in many fruits and vegetables, most notably apples. It is a dicarboxylic acid that is made by all living organisms, contributes to the pleasantly sour taste of fruits, and is used in food preparation and beverages for its sour taste.
Chemistry[edit]
Malic acid has two stereoisomeric forms (L- and D-enantiomers), though only the L-isomer exists naturally. The salts and esters of malic acid are known as malates. The malate anion is an intermediate in the citric acid cycle.
Biosynthesis[edit]
L-Malic acid is the naturally occurring form, whereas a mixture of L- and D-malic acid is produced synthetically. L-Malic acid is synthesized in the cytosol of plant cells by the enzyme malate dehydrogenase, a part of the oxidative decarboxylation process.
Uses[edit]
Malic acid contributes to the sourness of green apples. It is present in grapes and in most wines with concentrations sometimes as high as 5 g/l. It confers a tart taste to wine, although the amount decreases with increasing fruit ripeness. The process of malolactic fermentation converts malic acid to much milder lactic acid.
Safety[edit]
Malic acid, when added to food products, is denoted by E number E296. Malic acid is the source of extreme tartness in United States confectionery, the so-called extreme candy. It is also used with or in place of the less sour citric acid in sour sweets. These sweets are sometimes labeled with a warning that excessive consumption can cause irritation of the mouth. It is approved for use in the EU, US and Australia and New Zealand.