Quinine: Difference between revisions
No edit summary Tags: mobile edit mobile web edit |
CSV import |
||
| Line 1: | Line 1: | ||
{{Short description|An article about the use of quinine in medicine}} | |||
{{Infobox drug | |||
| name = Quinine | |||
| image = Quinine structure.png | |||
| width = 200px | |||
| alt = Quinine chemical structure | |||
| caption = Chemical structure of quinine | |||
| image2 = Tonic water uv.jpg | |||
| width2 = 200px | |||
| alt2 = Tonic water under UV light | |||
| caption2 = Tonic water glowing under UV light due to quinine | |||
}} | |||
'''Quinine''' is a medication used to treat [[malaria]] and [[babesiosis]]. It is a naturally occurring alkaloid derived from the bark of the [[Cinchona]] tree. Quinine has been used for centuries as an effective treatment for malaria, a disease caused by [[Plasmodium]] parasites transmitted through the bites of infected [[Anopheles]] mosquitoes. | |||
==History== | |||
The use of quinine dates back to the early 17th century when it was first introduced to Europe by Jesuit missionaries. The bark of the Cinchona tree, native to the Andean forests of South America, was used by indigenous people to treat fevers. The active ingredient, quinine, was isolated in 1820 by French researchers Pierre Joseph Pelletier and Joseph Bienaimé Caventou. | |||
== | ==Mechanism of Action== | ||
Quinine works by interfering with the growth and reproduction of the malaria parasite in the red blood cells. It inhibits the parasite's ability to digest hemoglobin, leading to the accumulation of toxic heme molecules, which ultimately kills the parasite. | |||
==Medical Uses== | |||
Quinine is primarily used to treat uncomplicated [[Plasmodium falciparum]] malaria. It is often used in combination with other antimalarial drugs to enhance efficacy and reduce the risk of resistance. Quinine is also used off-label to treat [[nocturnal leg cramps]], although this use is controversial due to potential side effects. | |||
==Side Effects== | |||
Common side effects of quinine include [[cinchonism]], which is characterized by symptoms such as tinnitus, headache, nausea, and visual disturbances. More severe side effects can include [[thrombocytopenia]], [[hemolytic anemia]], and [[arrhythmias]]. Due to these potential adverse effects, quinine use is generally limited to cases where other treatments are not available or suitable. | |||
== | ==Biosynthesis== | ||
[[File:Quinine Biosynthesis.png|thumb|Biosynthesis of quinine]] | |||
Quinine is biosynthesized in the Cinchona tree through a complex pathway involving several enzymatic steps. The process begins with the amino acid tryptophan and involves the formation of several intermediate compounds before the final quinine molecule is produced. | |||
Quinine is | ==Cultural and Other Uses== | ||
Quinine is also known for its use in [[tonic water]], where it is responsible for the drink's distinctive bitter taste. Under ultraviolet light, tonic water fluoresces due to the presence of quinine, as shown in the image above. | |||
== | ==Also see== | ||
* [[Malaria treatment]] | |||
* [[Cinchona]] | |||
* [[Antimalarial medication]] | |||
* [[Cinchonism]] | |||
==References== | |||
{{Reflist}} | |||
[[Category:Antimalarial agents]] | |||
[[Category:Alkaloids]] | |||
[[Category:World Health Organization essential medicines]] | |||
Latest revision as of 02:50, 11 December 2024
An article about the use of quinine in medicine
| Quinine | |
|---|---|
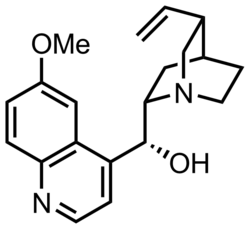
| |
| INN | |
| Drug class | |
| Routes of administration | |
| Pregnancy category | |
| Bioavailability | |
| Metabolism | |
| Elimination half-life | |
| Excretion | |
| Legal status | |
| CAS Number | |
| PubChem | |
| DrugBank | |
| ChemSpider | |
| KEGG | |
Quinine is a medication used to treat malaria and babesiosis. It is a naturally occurring alkaloid derived from the bark of the Cinchona tree. Quinine has been used for centuries as an effective treatment for malaria, a disease caused by Plasmodium parasites transmitted through the bites of infected Anopheles mosquitoes.
History[edit]
The use of quinine dates back to the early 17th century when it was first introduced to Europe by Jesuit missionaries. The bark of the Cinchona tree, native to the Andean forests of South America, was used by indigenous people to treat fevers. The active ingredient, quinine, was isolated in 1820 by French researchers Pierre Joseph Pelletier and Joseph Bienaimé Caventou.
Mechanism of Action[edit]
Quinine works by interfering with the growth and reproduction of the malaria parasite in the red blood cells. It inhibits the parasite's ability to digest hemoglobin, leading to the accumulation of toxic heme molecules, which ultimately kills the parasite.
Medical Uses[edit]
Quinine is primarily used to treat uncomplicated Plasmodium falciparum malaria. It is often used in combination with other antimalarial drugs to enhance efficacy and reduce the risk of resistance. Quinine is also used off-label to treat nocturnal leg cramps, although this use is controversial due to potential side effects.
Side Effects[edit]
Common side effects of quinine include cinchonism, which is characterized by symptoms such as tinnitus, headache, nausea, and visual disturbances. More severe side effects can include thrombocytopenia, hemolytic anemia, and arrhythmias. Due to these potential adverse effects, quinine use is generally limited to cases where other treatments are not available or suitable.
Biosynthesis[edit]

Quinine is biosynthesized in the Cinchona tree through a complex pathway involving several enzymatic steps. The process begins with the amino acid tryptophan and involves the formation of several intermediate compounds before the final quinine molecule is produced.
Cultural and Other Uses[edit]
Quinine is also known for its use in tonic water, where it is responsible for the drink's distinctive bitter taste. Under ultraviolet light, tonic water fluoresces due to the presence of quinine, as shown in the image above.
Also see[edit]
References[edit]
<references group="" responsive="1"></references>