Varenicline: Difference between revisions
Created page with "{{intro}} Varenicline is a partial agonist of the nicotinic acetylcholine receptor and is used to help in smoking cessation. {{livtox}} Varenicline has been associated with..." |
CSV import |
||
| Line 1: | Line 1: | ||
{{ | {{Short description|Medication used to treat nicotine addiction}} | ||
Varenicline | {{Drugbox | ||
| verifiedrevid = 477241123 | |||
| image = Varenicline.svg | |||
| image2 = Varenicline_ball-and-stick_model.png | |||
| width = 200 | |||
}} | |||
'''Varenicline''' is a medication used to treat [[nicotine addiction]]. It is marketed under the brand name '''Chantix''' in the United States and '''Champix''' in other countries. Varenicline is a prescription medication that helps people stop smoking by reducing withdrawal symptoms and decreasing the pleasurable effects of cigarettes and other tobacco products. | |||
Varenicline | |||
==Mechanism of action== | |||
Varenicline | Varenicline is a partial agonist of the [[nicotinic acetylcholine receptor]] subtype _4_2. This receptor is believed to play a key role in the addictive properties of nicotine. By partially stimulating these receptors, varenicline reduces cravings and withdrawal symptoms associated with smoking cessation. Additionally, it blocks nicotine from binding to these receptors, thereby reducing the rewarding effects of smoking. | ||
==Medical uses== | |||
Varenicline is | Varenicline is primarily used as an aid to smoking cessation. It is typically prescribed as part of a comprehensive program that includes counseling and support. The standard course of treatment lasts for 12 weeks, but it may be extended for an additional 12 weeks to increase the likelihood of long-term abstinence. | ||
==Side effects== | |||
Common side effects include nausea, | Common side effects of varenicline include nausea, headache, difficulty sleeping, and abnormal dreams. Some users may experience mood changes, depression, or suicidal thoughts. It is important for patients to be monitored for any changes in mood or behavior while taking this medication. | ||
==History== | |||
{{ | Varenicline was developed by [[Pfizer]] and was approved by the [[Food and Drug Administration]] (FDA) in 2006. It has since become a widely used treatment for smoking cessation, although its use has been subject to some controversy due to reports of psychiatric side effects. | ||
==Research== | |||
Ongoing research is examining the effectiveness of varenicline in combination with other smoking cessation therapies, as well as its potential use in treating other forms of addiction. Studies are also investigating the long-term safety and efficacy of varenicline. | |||
==Related pages== | |||
* [[Nicotine replacement therapy]] | |||
* [[Bupropion]] | |||
* [[Smoking cessation]] | |||
==References== | |||
{{Reflist}} | |||
[[Category:Smoking cessation]] | |||
[[Category:Pfizer brands]] | |||
[[Category:Nicotinic agonists]] | |||
Revision as of 20:56, 9 February 2025
Medication used to treat nicotine addiction
| Varenicline | |
|---|---|
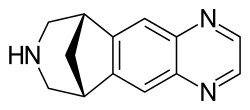
| |
| INN | |
| Drug class | |
| Routes of administration | |
| Pregnancy category | |
| Bioavailability | |
| Metabolism | |
| Elimination half-life | |
| Excretion | |
| Legal status | |
| CAS Number | |
| PubChem | |
| DrugBank | |
| ChemSpider | |
| KEGG | |
Varenicline is a medication used to treat nicotine addiction. It is marketed under the brand name Chantix in the United States and Champix in other countries. Varenicline is a prescription medication that helps people stop smoking by reducing withdrawal symptoms and decreasing the pleasurable effects of cigarettes and other tobacco products.
Mechanism of action
Varenicline is a partial agonist of the nicotinic acetylcholine receptor subtype _4_2. This receptor is believed to play a key role in the addictive properties of nicotine. By partially stimulating these receptors, varenicline reduces cravings and withdrawal symptoms associated with smoking cessation. Additionally, it blocks nicotine from binding to these receptors, thereby reducing the rewarding effects of smoking.
Medical uses
Varenicline is primarily used as an aid to smoking cessation. It is typically prescribed as part of a comprehensive program that includes counseling and support. The standard course of treatment lasts for 12 weeks, but it may be extended for an additional 12 weeks to increase the likelihood of long-term abstinence.
Side effects
Common side effects of varenicline include nausea, headache, difficulty sleeping, and abnormal dreams. Some users may experience mood changes, depression, or suicidal thoughts. It is important for patients to be monitored for any changes in mood or behavior while taking this medication.
History
Varenicline was developed by Pfizer and was approved by the Food and Drug Administration (FDA) in 2006. It has since become a widely used treatment for smoking cessation, although its use has been subject to some controversy due to reports of psychiatric side effects.
Research
Ongoing research is examining the effectiveness of varenicline in combination with other smoking cessation therapies, as well as its potential use in treating other forms of addiction. Studies are also investigating the long-term safety and efficacy of varenicline.
Related pages
References
<references group="" responsive="1"></references>