Cooperativity: Difference between revisions
CSV import |
CSV import |
||
| Line 37: | Line 37: | ||
[[Category:Biochemistry]] | [[Category:Biochemistry]] | ||
[[Category:Molecular Biology]] | [[Category:Molecular Biology]] | ||
<gallery> | |||
File:Hemoglobin_saturation_curve.svg | |||
File:CC-BY_icon.svg | |||
</gallery> | |||
Latest revision as of 02:09, 17 February 2025
Cooperativity
Cooperativity is a phenomenon observed in biochemical systems where the binding of a molecule to a target site affects the binding properties of additional molecules to the same system. This concept is crucial in understanding the behavior of enzymes and receptors in biological systems, particularly in the context of allosteric regulation and enzyme kinetics.
Mechanism of Cooperativity[edit]
Cooperativity occurs when the binding of a ligand to a protein affects the binding affinity of additional ligand molecules. This can be either positive or negative:
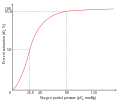
- Positive Cooperativity: The binding of the first ligand increases the affinity of the protein for subsequent ligands. This is often seen in proteins with multiple binding sites, such as hemoglobin, where the binding of oxygen to one heme group increases the affinity of the remaining heme groups for oxygen.
- Negative Cooperativity: The binding of the first ligand decreases the affinity of the protein for subsequent ligands. This can serve as a regulatory mechanism to prevent overactivity of a protein.
Models of Cooperativity[edit]
Several models have been proposed to explain cooperativity in proteins:
- The Concerted Model (MWC Model): Proposed by Monod, Wyman, and Changeux, this model suggests that all subunits of a protein are in the same state, either all in the tense (T) state or all in the relaxed (R) state. Ligand binding shifts the equilibrium towards the R state, increasing affinity.
- The Sequential Model (KNF Model): Proposed by Koshland, Némethy, and Filmer, this model suggests that subunits can change states independently. Ligand binding induces a conformational change in one subunit, which then influences neighboring subunits to increase their affinity for the ligand.
Examples of Cooperativity[edit]
- Hemoglobin: Hemoglobin is a classic example of positive cooperativity. It has four subunits, each capable of binding an oxygen molecule. The binding of oxygen to one subunit increases the affinity of the remaining subunits for oxygen, facilitating efficient oxygen uptake and release.
- Enzymes: Many enzymes exhibit cooperativity in substrate binding, which can be crucial for regulating metabolic pathways. For example, the enzyme aspartate transcarbamoylase (ATCase) shows positive cooperativity in its reaction with substrates.
Importance in Biology[edit]
Cooperativity is essential for the regulation of biological processes. It allows for fine-tuned responses to changes in ligand concentration, enabling cells to respond dynamically to environmental signals. This is particularly important in processes such as oxygen transport, signal transduction, and metabolic regulation.
Also see[edit]
| Biochemistry | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
This biochemistry related article is a stub.
|
| Molecular biology | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|