Thioester: Difference between revisions
CSV import |
CSV import |
||
| Line 26: | Line 26: | ||
{{stub}} | {{stub}} | ||
<gallery> | |||
File:Thioester-2D-A.svg|Thioester 2D Structure | |||
File:FormationofAmides.png|Formation of Amides | |||
File:NCL_mechanism.pdf|Native Chemical Ligation Mechanism | |||
File:FukuyamaCoupling.svg|Fukuyama Coupling | |||
File:Acetyl-CoA-2D.svg|Acetyl-CoA 2D Structure | |||
File:Thionoester_general_structure.png|Thionoester General Structure | |||
File:Methyl-thionobenzoate-2D-skeletal.png|Methyl Thionobenzoate 2D Skeletal | |||
File:Thionoester-from-thioacyl-chloride-2D-skeletal.png|Thionoester from Thioacyl Chloride 2D Skeletal | |||
File:Transesterification_of_Thionoesters.png|Transesterification of Thionoesters | |||
</gallery> | |||
Latest revision as of 21:44, 23 February 2025
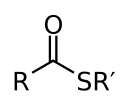
Thioester is a type of organic compound that is derived from carboxylic acids and thiols. They are the sulfur analogs of esters and are important in many biological processes, including the synthesis of fatty acids and polyketides.
Structure and Bonding[edit]
Thioesters are characterized by the presence of a sulfur atom, which replaces the oxygen atom found in regular esters. This results in a C-S-CO-R structure, where R represents any organic group. The C-S bond in thioesters is generally weaker than the C-O bond in esters, which has implications for their reactivity.
Synthesis[edit]
Thioesters can be synthesized in several ways. One common method is the reaction of a carboxylic acid with a thiol in the presence of a dehydrating agent. Another method is the reaction of an acyl chloride with a thiol. Thioesters can also be formed from alcohols and thiols using a Fischer esterification reaction.
Reactivity[edit]
Thioesters are more reactive than their oxygen counterparts due to the weaker C-S bond. They undergo many of the same reactions as esters, including hydrolysis, transesterification, and Claisen condensation. However, they also participate in unique reactions such as the Perkin reaction and the Dieckmann condensation.
Biological Importance[edit]
Thioesters play a crucial role in biology. They are involved in the synthesis of fatty acids and polyketides, and are key intermediates in the citric acid cycle. They also participate in protein synthesis and signal transduction.
See Also[edit]
|
|
|
-
Thioester 2D Structure
-
Formation of Amides
-
Native Chemical Ligation Mechanism
-
Fukuyama Coupling
-
Acetyl-CoA 2D Structure
-
Thionoester General Structure
-
Methyl Thionobenzoate 2D Skeletal
-
Thionoester from Thioacyl Chloride 2D Skeletal
-
Transesterification of Thionoesters