Daunorubicin: Difference between revisions
CSV import |
No edit summary |
||
| (3 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{ | {{Short description|An anthracycline antibiotic used in cancer treatment}} | ||
Daunorubicin is an anthracycline antibiotic that | {{Drugbox | ||
| verifiedfields = changed | |||
| verifiedrevid = 477002123 | |||
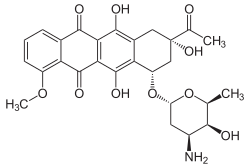
| IUPAC_name = (8S,10S)-8-acetyl-10-[(3-amino-2,3,6-trideoxy-_-L-lyxo-hexopyranosyl)oxy]-7,8,9,10-tetrahydro-6,8,11-trihydroxy-1-methoxy-5,12-naphthacenedione | |||
| image = Daunorubicin.svg | |||
| width = 200px | |||
}} | |||
[[File:Daunorubicin2DACS.svg|Daunorubicin 2D Structure|thumb]] | |||
[[File:Daunorubicin ball-and-stick.png|Daunorubicin Ball-and-Stick Model|thumb]] | |||
'''Daunorubicin''', also known as '''daunomycin''', is an anthracycline [[antibiotic]] that is primarily used in the treatment of certain types of [[cancer]], including [[acute myeloid leukemia]] (AML) and [[acute lymphoblastic leukemia]] (ALL). It is a chemotherapy agent that works by intercalating [[DNA]], thereby inhibiting the synthesis of [[nucleic acids]] and inducing apoptosis in rapidly dividing cells. | |||
==Mechanism of Action== | |||
Daunorubicin | Daunorubicin exerts its effects by intercalating between base pairs in the DNA helix, thereby disrupting the function of [[topoisomerase II]], an enzyme critical for DNA replication and repair. This interference prevents the proper unwinding of DNA, leading to breaks in the DNA strands and ultimately triggering cell death. Additionally, daunorubicin generates free radicals that cause further damage to cellular components. | ||
==Clinical Uses== | |||
Daunorubicin | Daunorubicin is primarily used in the treatment of: | ||
* [[Acute myeloid leukemia]] (AML) | |||
* [[Acute lymphoblastic leukemia]] (ALL) | |||
It is often used in combination with other chemotherapeutic agents, such as [[cytarabine]], to enhance its efficacy. The drug is administered intravenously, allowing it to rapidly reach systemic circulation and target cancerous cells throughout the body. | |||
==Side Effects== | |||
The use of daunorubicin is associated with several side effects, which can vary in severity. Common side effects include: | |||
* [[Myelosuppression]], leading to decreased production of blood cells | |||
* [[Nausea]] and [[vomiting]] | |||
* [[Alopecia]] (hair loss) | |||
* [[Cardiotoxicity]], which can lead to [[congestive heart failure]] | |||
Due to its potential to cause cardiotoxicity, the cumulative dose of daunorubicin is carefully monitored, and patients may undergo regular cardiac evaluations during treatment. | |||
==Pharmacokinetics== | |||
Daunorubicin is metabolized primarily in the liver and is excreted in the bile and urine. Its half-life is approximately 18.5 hours, allowing for effective dosing schedules in chemotherapy regimens. The drug's lipophilic nature facilitates its penetration into tissues, including the [[central nervous system]], although its efficacy in treating [[central nervous system leukemia]] is limited. | |||
==History== | |||
Daunorubicin was first isolated from the bacterium ''Streptomyces peucetius'' in the 1960s. It was one of the first anthracyclines to be used in clinical practice and paved the way for the development of other related compounds, such as [[doxorubicin]]. | |||
==Related pages== | |||
* [[Doxorubicin]] | |||
* [[Chemotherapy]] | |||
* [[Leukemia]] | |||
* [[Antibiotics]] | |||
{{chemotherapy}} | |||
[[Category:Chemotherapy agents]] | |||
[[Category:Anthracyclines]] | |||
[[Category:Antibiotics]] | |||
Latest revision as of 12:13, 21 March 2025
An anthracycline antibiotic used in cancer treatment
| Daunorubicin | |
|---|---|
| |
| INN | |
| Drug class | |
| Routes of administration | |
| Pregnancy category | |
| Bioavailability | |
| Metabolism | |
| Elimination half-life | |
| Excretion | |
| Legal status | |
| CAS Number | |
| PubChem | |
| DrugBank | |
| ChemSpider | |
| KEGG | |
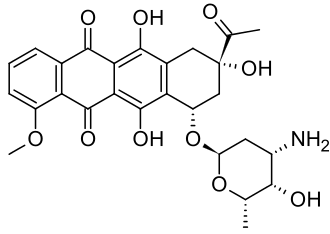

Daunorubicin, also known as daunomycin, is an anthracycline antibiotic that is primarily used in the treatment of certain types of cancer, including acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL). It is a chemotherapy agent that works by intercalating DNA, thereby inhibiting the synthesis of nucleic acids and inducing apoptosis in rapidly dividing cells.
Mechanism of Action[edit]
Daunorubicin exerts its effects by intercalating between base pairs in the DNA helix, thereby disrupting the function of topoisomerase II, an enzyme critical for DNA replication and repair. This interference prevents the proper unwinding of DNA, leading to breaks in the DNA strands and ultimately triggering cell death. Additionally, daunorubicin generates free radicals that cause further damage to cellular components.
Clinical Uses[edit]
Daunorubicin is primarily used in the treatment of:
- Acute myeloid leukemia (AML)
- Acute lymphoblastic leukemia (ALL)
It is often used in combination with other chemotherapeutic agents, such as cytarabine, to enhance its efficacy. The drug is administered intravenously, allowing it to rapidly reach systemic circulation and target cancerous cells throughout the body.
Side Effects[edit]
The use of daunorubicin is associated with several side effects, which can vary in severity. Common side effects include:
- Myelosuppression, leading to decreased production of blood cells
- Nausea and vomiting
- Alopecia (hair loss)
- Cardiotoxicity, which can lead to congestive heart failure
Due to its potential to cause cardiotoxicity, the cumulative dose of daunorubicin is carefully monitored, and patients may undergo regular cardiac evaluations during treatment.
Pharmacokinetics[edit]
Daunorubicin is metabolized primarily in the liver and is excreted in the bile and urine. Its half-life is approximately 18.5 hours, allowing for effective dosing schedules in chemotherapy regimens. The drug's lipophilic nature facilitates its penetration into tissues, including the central nervous system, although its efficacy in treating central nervous system leukemia is limited.
History[edit]
Daunorubicin was first isolated from the bacterium Streptomyces peucetius in the 1960s. It was one of the first anthracyclines to be used in clinical practice and paved the way for the development of other related compounds, such as doxorubicin.
Related pages[edit]
| Chemotherapy | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
This Chemotherapy related article is a stub.
|