DNA glycosylase
DNA glycosylases are a family of enzymes that play a crucial role in the repair of DNA by initiating the base excision repair (BER) pathway. These enzymes recognize and remove damaged or inappropriate bases from DNA, thereby preventing mutations that could lead to cancer, aging, and various genetic disorders. DNA glycosylases have been identified in a wide range of organisms, from bacteria to humans, highlighting their importance in maintaining genomic stability.
Function[edit]
DNA glycosylases function by flipping the damaged base out of the DNA helix and into the enzyme's active site. Once the damaged base is removed, the site is left apurinic/apyrimidinic (AP site), which is then recognized and processed by other enzymes in the BER pathway. This process involves several steps, including the removal of the sugar-phosphate backbone, filling in the gap with the correct base, and sealing the nick in the DNA backbone. The specificity of DNA glycosylases for different types of damage is a key aspect of their function, with different enzymes recognizing specific types of damaged bases, such as those caused by oxidative stress, alkylation, or deamination.
Types of DNA Glycosylases[edit]
There are several types of DNA glycosylases, each with specificity for different damaged or inappropriate bases. Some of the well-known DNA glycosylases include:
- Uracil-DNA glycosylase (UNG): Recognizes and removes uracil from DNA, which can arise through deamination of cytosine or incorporation during DNA replication.
- 8-Oxoguanine DNA glycosylase (OGG1): Targets 8-oxoguanine, a common lesion formed by oxidative damage, which can mispair with adenine during replication.
- Thymine DNA glycosylase (TDG): Removes thymine glycol and other oxidative thymine lesions, as well as thymine mispaired with guanine.
- MutY DNA glycosylase (MUTYH): Removes adenines misincorporated opposite 8-oxoguanine, preventing G:C to T:A transversion mutations.
Clinical Significance[edit]
Mutations in the genes encoding DNA glycosylases can lead to a predisposition to cancer and other diseases. For example, mutations in the MUTYH gene are associated with MUTYH-associated polyposis (MAP), a condition that increases the risk of developing colorectal cancer. Understanding the mechanisms of action and regulation of DNA glycosylases is crucial for developing novel therapeutic strategies for treating diseases associated with DNA damage and repair defects.
Research and Therapeutic Applications[edit]
Research into DNA glycosylases has led to the development of inhibitors and synthetic lethality strategies for cancer therapy. By targeting specific DNA repair pathways in cancer cells, which often rely on these mechanisms due to their high rate of DNA damage, it is possible to selectively kill cancer cells while sparing normal cells. Additionally, understanding the role of DNA glycosylases in aging and neurodegenerative diseases could lead to the development of interventions aimed at promoting genomic stability and healthy aging.
See Also[edit]

This article is a molecular biology stub. You can help WikiMD by expanding it!
-
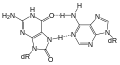
Uracil base glycosidase
-
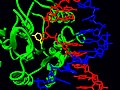
PDB structure 2j8x
-
8-oxoG forming Hoogsteen base pair with dA
-
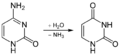
Deamination of cytosine to uracil
Ad. Transform your life with W8MD's Budget GLP-1 injections from $75


W8MD offers a medical weight loss program to lose weight in Philadelphia. Our physician-supervised medical weight loss provides:
- Weight loss injections in NYC (generic and brand names):
- Zepbound / Mounjaro, Wegovy / Ozempic, Saxenda
- Most insurances accepted or discounted self-pay rates. We will obtain insurance prior authorizations if needed.
- Generic GLP1 weight loss injections from $75 for the starting dose.
- Also offer prescription weight loss medications including Phentermine, Qsymia, Diethylpropion, Contrave etc.
NYC weight loss doctor appointmentsNYC weight loss doctor appointments
Start your NYC weight loss journey today at our NYC medical weight loss and Philadelphia medical weight loss clinics.
- Call 718-946-5500 to lose weight in NYC or for medical weight loss in Philadelphia 215-676-2334.
- Tags:NYC medical weight loss, Philadelphia lose weight Zepbound NYC, Budget GLP1 weight loss injections, Wegovy Philadelphia, Wegovy NYC, Philadelphia medical weight loss, Brookly weight loss and Wegovy NYC
|
WikiMD's Wellness Encyclopedia |
| Let Food Be Thy Medicine Medicine Thy Food - Hippocrates |
Medical Disclaimer: WikiMD is not a substitute for professional medical advice. The information on WikiMD is provided as an information resource only, may be incorrect, outdated or misleading, and is not to be used or relied on for any diagnostic or treatment purposes. Please consult your health care provider before making any healthcare decisions or for guidance about a specific medical condition. WikiMD expressly disclaims responsibility, and shall have no liability, for any damages, loss, injury, or liability whatsoever suffered as a result of your reliance on the information contained in this site. By visiting this site you agree to the foregoing terms and conditions, which may from time to time be changed or supplemented by WikiMD. If you do not agree to the foregoing terms and conditions, you should not enter or use this site. See full disclaimer.
Credits:Most images are courtesy of Wikimedia commons, and templates, categories Wikipedia, licensed under CC BY SA or similar.
Translate this page: - East Asian
中文,
日本,
한국어,
South Asian
हिन्दी,
தமிழ்,
తెలుగు,
Urdu,
ಕನ್ನಡ,
Southeast Asian
Indonesian,
Vietnamese,
Thai,
မြန်မာဘာသာ,
বাংলা
European
español,
Deutsch,
français,
Greek,
português do Brasil,
polski,
română,
русский,
Nederlands,
norsk,
svenska,
suomi,
Italian
Middle Eastern & African
عربى,
Turkish,
Persian,
Hebrew,
Afrikaans,
isiZulu,
Kiswahili,
Other
Bulgarian,
Hungarian,
Czech,
Swedish,
മലയാളം,
मराठी,
ਪੰਜਾਬੀ,
ગુજરાતી,
Portuguese,
Ukrainian