Asymmetric carbon
Asymmetric Carbon
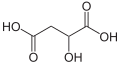
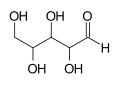
An asymmetric carbon (also known as a chiral carbon) is a carbon atom that is attached to four different types of atoms or groups of atoms. This configuration results in molecules that have a unique property called chirality, which is the quality of being non-superimposable on their mirror images. This property is significant in many areas of science and technology, including chemistry, biochemistry, pharmaceuticals, and materials science.
Overview[edit]
The concept of asymmetric carbon was first introduced by Louis Pasteur, a French chemist and microbiologist, in the 19th century. Pasteur discovered that certain organic compounds could exist in two different forms that were mirror images of each other, a phenomenon he attributed to the presence of asymmetric carbon atoms.
Structure and Properties[edit]
An asymmetric carbon atom is sp3 hybridized, meaning it forms four sigma bonds with four different atoms or groups of atoms. These four groups can be arranged in two different ways around the carbon atom, resulting in two different spatial arrangements or stereoisomers. These stereoisomers are mirror images of each other and are known as enantiomers.
The presence of an asymmetric carbon atom in a molecule can give rise to interesting and important properties. For example, the two enantiomers of a molecule may have different chemical reactivities or biological activities. This is particularly important in the field of pharmaceuticals, where one enantiomer of a drug may be therapeutically active while the other is not, or may even be harmful.
Applications[edit]
Asymmetric carbon atoms are found in many important molecules in nature, including amino acids, the building blocks of proteins, and sugars, important energy sources and structural components of living organisms. The ability to synthesize and manipulate molecules with asymmetric carbon atoms is therefore of great importance in the fields of organic chemistry and biochemistry.
In the pharmaceutical industry, the synthesis of drugs often involves the creation of molecules with one or more asymmetric carbon atoms. The ability to selectively produce one enantiomer of a drug over the other can have significant implications for the drug's safety and efficacy.
See Also[edit]
-
Asymmetric_carbon
-
Asymmetric_carbon
-
Asymmetric_carbon
-
Asymmetric_carbon
Ad. Transform your life with W8MD's Budget GLP-1 injections from $49.99


W8MD offers a medical weight loss program to lose weight in Philadelphia. Our physician-supervised medical weight loss provides:
- Weight loss injections in NYC (generic and brand names):
- Zepbound / Mounjaro, Wegovy / Ozempic, Saxenda
- Most insurances accepted or discounted self-pay rates. We will obtain insurance prior authorizations if needed.
- Generic GLP1 weight loss injections from $49.99 for the starting dose of Semaglutide and $65.00 for Tirzepatide.
- Also offer prescription weight loss medications including Phentermine, Qsymia, Diethylpropion, Contrave etc.
NYC weight loss doctor appointmentsNYC weight loss doctor appointments
Start your NYC weight loss journey today at our NYC medical weight loss and Philadelphia medical weight loss clinics.
- Call 718-946-5500 to lose weight in NYC or for medical weight loss in Philadelphia 215-676-2334.
- Tags:NYC medical weight loss, Philadelphia lose weight Zepbound NYC, Budget GLP1 weight loss injections, Wegovy Philadelphia, Wegovy NYC, Philadelphia medical weight loss, Brookly weight loss and Wegovy NYC
|
WikiMD's Wellness Encyclopedia |
| Let Food Be Thy Medicine Medicine Thy Food - Hippocrates |
Medical Disclaimer: WikiMD is not a substitute for professional medical advice. The information on WikiMD is provided as an information resource only, may be incorrect, outdated or misleading, and is not to be used or relied on for any diagnostic or treatment purposes. Please consult your health care provider before making any healthcare decisions or for guidance about a specific medical condition. WikiMD expressly disclaims responsibility, and shall have no liability, for any damages, loss, injury, or liability whatsoever suffered as a result of your reliance on the information contained in this site. By visiting this site you agree to the foregoing terms and conditions, which may from time to time be changed or supplemented by WikiMD. If you do not agree to the foregoing terms and conditions, you should not enter or use this site. See full disclaimer.
Credits:Most images are courtesy of Wikimedia commons, and templates, categories Wikipedia, licensed under CC BY SA or similar.
Translate this page: - East Asian
中文,
日本,
한국어,
South Asian
हिन्दी,
தமிழ்,
తెలుగు,
Urdu,
ಕನ್ನಡ,
Southeast Asian
Indonesian,
Vietnamese,
Thai,
မြန်မာဘာသာ,
বাংলা
European
español,
Deutsch,
français,
Greek,
português do Brasil,
polski,
română,
русский,
Nederlands,
norsk,
svenska,
suomi,
Italian
Middle Eastern & African
عربى,
Turkish,
Persian,
Hebrew,
Afrikaans,
isiZulu,
Kiswahili,
Other
Bulgarian,
Hungarian,
Czech,
Swedish,
മലയാളം,
मराठी,
ਪੰਜਾਬੀ,
ગુજરાતી,
Portuguese,
Ukrainian