Aromatization: Difference between revisions
CSV import |
CSV import |
||
| Line 1: | Line 1: | ||
== Aromatization == | == Aromatization == | ||
Aromatization is a chemical process by which an aliphatic compound is converted into an aromatic compound. This transformation is significant in both organic chemistry and biochemistry, as it involves the formation of stable aromatic rings, which are prevalent in many natural and synthetic compounds. | |||
[[File:MeC6H11toPhMe.png|Aromatization of methylcyclohexane to toluene|thumb|right]] | |||
== Chemical Process == | |||
Aromatization typically involves the dehydrogenation of cyclic alkanes to form aromatic hydrocarbons. This process can be catalyzed by various agents, including metals and metal oxides. For example, the conversion of [[methylcyclohexane]] to [[toluene]] is a classic example of aromatization, where hydrogen atoms are removed to form the aromatic ring structure. | |||
Aromatization | [[File:MeC5H9toPhH.png|Aromatization of methylcyclopentane to benzene|thumb|left]] | ||
In the laboratory, aromatization can be achieved using specific reagents such as [[DDQ]] (2,3-dichloro-5,6-dicyano-1,4-benzoquinone), which facilitates the dehydrogenation of cyclic compounds. The [[DDQ aromatization rearrangement]] is a notable reaction in this context. | |||
== Biological Aromatization == | |||
In biological systems, aromatization is a crucial step in the biosynthesis of [[estrogens]] from [[androgens]]. The enzyme [[aromatase]] catalyzes the conversion of [[testosterone]] to [[estradiol]], a process essential for the regulation of reproductive and other physiological functions. | |||
[[File:Testosterone_estradiol_conversion.png|Conversion of testosterone to estradiol|thumb|right]] | |||
== Industrial Applications == | |||
Aromatization is also important in the petrochemical industry, where it is used to convert aliphatic hydrocarbons into aromatic compounds, which are valuable as chemical feedstocks and in the production of [[fuels]]. The process is often carried out in the presence of catalysts such as [[platinum]] or [[molybdenum]] on alumina supports. | |||
== Related Reactions == | |||
Several related chemical reactions involve aromatization as a key step. The [[Semmler-Wolff reaction]] is one such example, where cyclohexanones are converted to phenols. | |||
[[File:Semmler-Wolff_reaction.svg|Semmler-Wolff reaction|thumb|left]] | |||
Another example is the aromatization of [[tetrahydronaphthalenedione]] to form naphthalene derivatives, which are important in the synthesis of dyes and pharmaceuticals. | |||
[[File:Tetrahydronaphthalenedione.png|Aromatization of tetrahydronaphthalenedione|thumb|right]] | |||
== Related Pages == | == Related Pages == | ||
* [[Aromaticity]] | |||
* [[Dehydrogenation]] | |||
* [[Aromatase]] | |||
* [[Estrogen]] | * [[Estrogen]] | ||
* [[ | * [[Petrochemical industry]] | ||
== External Links == | |||
* [Aromatization on Wikimedia Commons](https://commons.wikimedia.org/wiki/Category:Aromatization) | |||
{{Portal|Chemistry}} | |||
[[Category:Organic reactions]] | |||
[[Category:Aromatic compounds]] | |||
[[Category:Biochemistry]] | [[Category:Biochemistry]] | ||
Latest revision as of 18:53, 23 March 2025
Aromatization[edit]
Aromatization is a chemical process by which an aliphatic compound is converted into an aromatic compound. This transformation is significant in both organic chemistry and biochemistry, as it involves the formation of stable aromatic rings, which are prevalent in many natural and synthetic compounds.

Chemical Process[edit]
Aromatization typically involves the dehydrogenation of cyclic alkanes to form aromatic hydrocarbons. This process can be catalyzed by various agents, including metals and metal oxides. For example, the conversion of methylcyclohexane to toluene is a classic example of aromatization, where hydrogen atoms are removed to form the aromatic ring structure.

In the laboratory, aromatization can be achieved using specific reagents such as DDQ (2,3-dichloro-5,6-dicyano-1,4-benzoquinone), which facilitates the dehydrogenation of cyclic compounds. The DDQ aromatization rearrangement is a notable reaction in this context.
Biological Aromatization[edit]
In biological systems, aromatization is a crucial step in the biosynthesis of estrogens from androgens. The enzyme aromatase catalyzes the conversion of testosterone to estradiol, a process essential for the regulation of reproductive and other physiological functions.
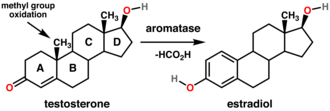
Industrial Applications[edit]
Aromatization is also important in the petrochemical industry, where it is used to convert aliphatic hydrocarbons into aromatic compounds, which are valuable as chemical feedstocks and in the production of fuels. The process is often carried out in the presence of catalysts such as platinum or molybdenum on alumina supports.
Related Reactions[edit]
Several related chemical reactions involve aromatization as a key step. The Semmler-Wolff reaction is one such example, where cyclohexanones are converted to phenols.

Another example is the aromatization of tetrahydronaphthalenedione to form naphthalene derivatives, which are important in the synthesis of dyes and pharmaceuticals.

Related Pages[edit]
External Links[edit]
- [Aromatization on Wikimedia Commons](https://commons.wikimedia.org/wiki/Category:Aromatization)
Lua error: bad argument #2 to 'title.new' (unrecognized namespace name 'Portal').