Aminoacetonitrile: Difference between revisions
CSV import |
CSV import |
||
| Line 27: | Line 27: | ||
{{medicine-stub}} | {{medicine-stub}} | ||
{{No image}} | {{No image}} | ||
== Aminoacetonitrile gallery == | |||
<gallery> | |||
File:Aminoacetonitrile.svg|Aminoacetonitrile.svg | |||
File:Aminoacetonitril.svg|Aminoacetonitril.svg | |||
File:Aminoacetonitrile-3D-balls.png|Aminoacetonitrile-3D-balls.png | |||
File:Aminoacetonitrile-3D-spacefill.png|Aminoacetonitrile-3D-spacefill.png | |||
</gallery> | |||
Revision as of 05:16, 3 March 2025
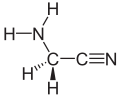
Aminoacetonitrile is an organic compound with the formula NH2CH2CN. This colorless liquid is a valuable reagent in the synthesis of various organic compounds, including many biologically active compounds. It is derived from acetonitrile by nucleophilic substitution with ammonia, a process that exploits the high reactivity of the nitrile group.
Structure and properties
Aminoacetonitrile is a simple organic molecule with a linear structure. The molecule consists of a nitrile group (-CN) and an amino group (-NH2) attached to a central carbon atom. The nitrile group is a strong electron-withdrawing group, which makes the molecule polar and gives it a high dipole moment. The amino group, on the other hand, is a strong electron-donating group, which can form hydrogen bonds with other molecules.
Synthesis
Aminoacetonitrile can be synthesized from acetonitrile by nucleophilic substitution with ammonia. This reaction is typically carried out in a solvent such as ethanol or water, and may require the use of a catalyst to increase the reaction rate. The product is usually purified by distillation.
Applications
Aminoacetonitrile is a versatile reagent in organic synthesis. It can be used to prepare a wide range of biologically active compounds, including antibiotics, antiviral drugs, and antifungal agents. It is also used in the synthesis of amino acids, peptides, and proteins.
Safety
Aminoacetonitrile is a toxic compound and should be handled with care. It can cause irritation to the skin, eyes, and respiratory tract. Ingestion or inhalation of large amounts can lead to serious health effects, including damage to the nervous system and kidneys.
See also
Aminoacetonitrile gallery
-
Aminoacetonitrile.svg
-
Aminoacetonitril.svg
-
Aminoacetonitrile-3D-balls.png
-
Aminoacetonitrile-3D-spacefill.png