RG7795: Difference between revisions
From WikiMD's Wellness Encyclopedia
CSV import Tags: mobile edit mobile web edit |
CSV import |
||
| Line 53: | Line 53: | ||
[[Category:Experimental drugs]] | [[Category:Experimental drugs]] | ||
{{dictionary-stub1}} | {{dictionary-stub1}} | ||
== RG7795 == | |||
<gallery> | |||
File:RG-7795.svg|RG-7795 | |||
File:Isatoribine.svg|Isatoribine | |||
</gallery> | |||
Latest revision as of 01:50, 20 February 2025
This article has multiple issues. Please help improve it or discuss these issues on the talk page. (Learn how and when to remove these messages)
|
| RG7795 | |
|---|---|
| [[File:|frameless|220px|alt=|]] | |
| INN | |
| Drug class | |
| Routes of administration | |
| Pregnancy category | |
| Bioavailability | |
| Metabolism | |
| Elimination half-life | |
| Excretion | |
| Legal status | Investigational |
| CAS Number | 1174920-78-5 |
| PubChem | |
| DrugBank | |
| ChemSpider | |
| KEGG | |
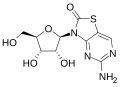
RG7795 (previously ANA773) is an antiviral drug candidate that as of 2015 had been in Phase II trials in hepatitis B.<ref name=Adis>
RG 7795(link). {{{website}}}. AdisInsight.
</ref> It is an orally-available prodrug of isatoribine,<ref name=Funk>,
Tickling the TLR7 to cure viral hepatitis., Journal of Translational Medicine, Vol. 12, pp. 129, DOI: 10.1186/1479-5876-12-129, PMID: 24884741, PMC: 4039542,</ref> that was under development by Anadys Pharmaceuticals when it was acquired by Roche in 2011.<ref>
,
Inovio Goes It Alone on Hepatitis B Immunotherapy Vaccine as Roche Ends Collaboration Full text, Genetic Engineering News, August 3, 2016,
</ref> Its active metabolite is an [agonist]] of TLR7; activation of TLR7 causes secretion of endogenous type 1 interferons, which have antiviral activity.<ref name=Funk/>
References[edit]
<references/>
RG7795[edit]
-
RG-7795
-
Isatoribine