Altrose: Difference between revisions
CSV import |
CSV import |
||
| Line 4: | Line 4: | ||
File:D-Altrose_Haworth.svg|D-Altrose Haworth projection | File:D-Altrose_Haworth.svg|D-Altrose Haworth projection | ||
</gallery> | </gallery> | ||
== Altrose == | |||
'''Altrose''' is a type of [[monosaccharide]], specifically an [[aldohexose]], which means it is a six-carbon sugar with an aldehyde group. It is one of the rare sugars and is not commonly found in nature. Altrose is an epimer of [[allose]], differing in the configuration around one specific carbon atom. | |||
== Structure == | |||
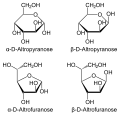
Altrose has the chemical formula C_H__O_. It is a stereoisomer of glucose, meaning it has the same molecular formula but a different three-dimensional arrangement of atoms. The structure of altrose can be represented in both its open-chain form and its cyclic form. In the open-chain form, altrose has an aldehyde group at the first carbon, and hydroxyl groups attached to the other carbons. | |||
In its cyclic form, altrose can form a six-membered ring called a pyranose. The cyclic form is more stable and is the predominant form in aqueous solutions. The specific configuration of altrose is determined by the orientation of the hydroxyl groups on the asymmetric carbon atoms. | |||
== Stereochemistry == | |||
Altrose is one of the eight D-aldohexoses, which also include [[glucose]], [[mannose]], [[galactose]], [[gulose]], [[idose]], [[talose]], and [[allose]]. The D- and L- configurations refer to the orientation of the hydroxyl group on the chiral carbon farthest from the aldehyde group. D-altrose is the enantiomer of L-altrose. | |||
== Biological Role == | |||
Altrose is not commonly found in nature and does not play a significant role in biological systems. It is not a major component of any known metabolic pathways in humans or other organisms. However, like other rare sugars, altrose can be synthesized in the laboratory and studied for its potential applications in medicine and biotechnology. | |||
== Synthesis == | |||
Altrose can be synthesized through various chemical methods, including the Kiliani-Fischer synthesis, which involves the chain extension of an aldose sugar. It can also be produced through the epimerization of other sugars, such as allose, using specific enzymes or chemical catalysts. | |||
== Applications == | |||
While altrose itself is not widely used, the study of rare sugars like altrose is important in the field of carbohydrate chemistry. Understanding the properties and reactions of these sugars can lead to the development of new pharmaceuticals and other biotechnological applications. | |||
== Related pages == | |||
* [[Monosaccharide]] | |||
* [[Aldohexose]] | |||
* [[Stereochemistry]] | |||
* [[Epimer]] | |||
* [[Carbohydrate chemistry]] | |||
{{Monosaccharides}} | |||
[[Category:Monosaccharides]] | |||
[[Category:Aldohexoses]] | |||
Latest revision as of 00:34, 19 February 2025
-
Altrose linear structure
-
Altrose
-
D-Altrose Haworth projection
Altrose[edit]
Altrose is a type of monosaccharide, specifically an aldohexose, which means it is a six-carbon sugar with an aldehyde group. It is one of the rare sugars and is not commonly found in nature. Altrose is an epimer of allose, differing in the configuration around one specific carbon atom.
Structure[edit]
Altrose has the chemical formula C_H__O_. It is a stereoisomer of glucose, meaning it has the same molecular formula but a different three-dimensional arrangement of atoms. The structure of altrose can be represented in both its open-chain form and its cyclic form. In the open-chain form, altrose has an aldehyde group at the first carbon, and hydroxyl groups attached to the other carbons.
In its cyclic form, altrose can form a six-membered ring called a pyranose. The cyclic form is more stable and is the predominant form in aqueous solutions. The specific configuration of altrose is determined by the orientation of the hydroxyl groups on the asymmetric carbon atoms.
Stereochemistry[edit]
Altrose is one of the eight D-aldohexoses, which also include glucose, mannose, galactose, gulose, idose, talose, and allose. The D- and L- configurations refer to the orientation of the hydroxyl group on the chiral carbon farthest from the aldehyde group. D-altrose is the enantiomer of L-altrose.
Biological Role[edit]
Altrose is not commonly found in nature and does not play a significant role in biological systems. It is not a major component of any known metabolic pathways in humans or other organisms. However, like other rare sugars, altrose can be synthesized in the laboratory and studied for its potential applications in medicine and biotechnology.
Synthesis[edit]
Altrose can be synthesized through various chemical methods, including the Kiliani-Fischer synthesis, which involves the chain extension of an aldose sugar. It can also be produced through the epimerization of other sugars, such as allose, using specific enzymes or chemical catalysts.
Applications[edit]
While altrose itself is not widely used, the study of rare sugars like altrose is important in the field of carbohydrate chemistry. Understanding the properties and reactions of these sugars can lead to the development of new pharmaceuticals and other biotechnological applications.