Nitrogenase: Difference between revisions
CSV import |
CSV import |
||
| Line 24: | Line 24: | ||
{{stub}} | {{stub}} | ||
{{dictionary-stub1}} | {{dictionary-stub1}} | ||
<gallery> | |||
File:Nitrogenase.png|Nitrogenase | |||
File:FeMoco_cluster.svg|FeMoco cluster | |||
File:Correct_Cartoon_Nitrogenase_with_Active_Sites_Highlighted.png|Cartoon of Nitrogenase with Active Sites Highlighted | |||
File:Cartoon_Nitrogenase_with_Active_Site_Magnified.png|Cartoon Nitrogenase with Active Site Magnified | |||
File:Corrected_Nitrogenase_Active_Sites.jpg|Corrected Nitrogenase Active Sites | |||
File:Lowe-Thorneley_Kinetic_Model.jpg|Lowe-Thorneley Kinetic Model | |||
File:N2-fixation-mech.jpg|N2 fixation mechanism | |||
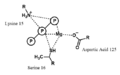
File:Binding_of_MgATP_by_nitrogenase.png|Binding of MgATP by nitrogenase | |||
</gallery> | |||
Revision as of 12:08, 18 February 2025
Nitrogenase is an enzyme that is produced by certain microorganisms, such as bacteria and archaea. This enzyme is responsible for the conversion of nitrogen gas (N2) into ammonia (NH3), a process known as nitrogen fixation. Nitrogenase is a complex enzyme that requires a significant amount of energy to function, and it is sensitive to the presence of oxygen.
Structure
Nitrogenase is composed of two proteins: the dinitrogenase reductase (Fe protein) and the dinitrogenase (MoFe protein). The Fe protein is a homodimer with an ATP-binding site and a [4Fe-4S] cluster. The MoFe protein is a heterotetramer that contains two types of metal clusters: the P-cluster and the FeMo-cofactor.
Function
The primary function of nitrogenase is to catalyze the reduction of nitrogen (N2) to ammonia (NH3). This process is critical for life on Earth, as nitrogen is a key component of amino acids, proteins, and nucleic acids. However, most organisms cannot use atmospheric nitrogen directly and rely on nitrogenase to convert it into a usable form.
Mechanism
The mechanism of nitrogenase is complex and not fully understood. However, it is known that the reaction requires a significant amount of energy, which is provided by the hydrolysis of ATP. The reaction also involves the transfer of electrons from the Fe protein to the MoFe protein.
Regulation
The activity of nitrogenase is tightly regulated by the cell. The enzyme is sensitive to oxygen, which can damage its metal clusters. Therefore, nitrogen-fixing organisms have developed various strategies to protect nitrogenase from oxygen, such as producing the enzyme in specialized cells or compartments that are devoid of oxygen.
See also
|
|
|
-
Nitrogenase
-
FeMoco cluster
-
Cartoon of Nitrogenase with Active Sites Highlighted
-
Cartoon Nitrogenase with Active Site Magnified
-
Corrected Nitrogenase Active Sites
-
Lowe-Thorneley Kinetic Model
-
N2 fixation mechanism
-
Binding of MgATP by nitrogenase