Carboximidate: Difference between revisions
CSV import Tags: mobile edit mobile web edit |
CSV import |
||
| Line 24: | Line 24: | ||
{{Chemistry-stub}} | {{Chemistry-stub}} | ||
<gallery> | |||
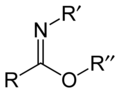
File:Imidate-2D-skeletal.png|Carboximidate | |||
File:Pinner_Reaktion_Grundmecha_Version_1-Seite001.svg|Pinner Reaction Mechanism | |||
File:Amidate-resonance-2D-skeletal.png|Amidate Resonance Structure | |||
File:Pinner_Übersicht_Version_2.svg|Pinner Reaction Overview | |||
File:Chapman_rearrangement.svg|Chapman Rearrangement | |||
File:Benzyl_2,2,2-trichloroacetimidate.svg|Benzyl 2,2,2-trichloroacetimidate | |||
</gallery> | |||
Latest revision as of 11:00, 18 February 2025
Carboximidate refers to a class of chemical compounds characterized by the presence of a carboximidate group. This group consists of a carbon atom double-bonded to an oxygen atom (carbonyl group) and single-bonded to a nitrogen atom, which in turn is typically bonded to another substituent. The general formula for a carboximidate ion is R-C(=O)NHR', where R and R' can be hydrogen atoms, alkyl, aryl, or other substituents. Carboximidates are closely related to carboxylic acids and amides, differing primarily in the substitution of the oxygen atom in the hydroxyl group of a carboxylic acid with a nitrogen atom in carboximidates.
Synthesis[edit]
Carboximidates are synthesized through several methods, with one common approach being the reaction of carboxylic acids with amines in the presence of activating agents. This process typically involves the dehydration of the carboxylic acid and amine to form the carboximidate. Another method involves the reaction of nitriles with alcohols, which can also yield carboximidate esters.
Properties[edit]
Carboximidates exhibit properties that are intermediate between those of carboxylic acids and amides. They are generally more nucleophilic than amides due to the presence of the nitrogen atom, which can donate electron density to the carbonyl carbon, making it more reactive towards electrophiles. This nucleophilicity is influenced by the substituents attached to the nitrogen and the carbonyl carbon.
Applications[edit]
Carboximidates find applications in various fields, including organic synthesis and pharmaceuticals. In organic chemistry, they are used as intermediates in the synthesis of a wide range of compounds, including heterocycles, alkaloids, and other functionalized molecules. In the pharmaceutical industry, certain carboximidate derivatives serve as active pharmaceutical ingredients (APIs) due to their biological activity, which can be attributed to their ability to interact with biological macromolecules in a specific manner.
Safety and Environmental Considerations[edit]
Like many chemical compounds, the safety and environmental impact of carboximidates depend on their specific chemical structure and the conditions under which they are used or disposed of. Proper handling, storage, and disposal procedures should be followed to minimize any potential health and environmental risks.
See Also[edit]
-
Carboximidate
-
Pinner Reaction Mechanism
-
Amidate Resonance Structure
-
Pinner Reaction Overview
-
Chapman Rearrangement
-
Benzyl 2,2,2-trichloroacetimidate