1,4-Naphthoquinone: Difference between revisions
CSV import |
CSV import |
||
| Line 42: | Line 42: | ||
File:1,4-Naphthoquinone-3D-balls.png|3D ball-and-stick model of 1,4-Naphthoquinone | File:1,4-Naphthoquinone-3D-balls.png|3D ball-and-stick model of 1,4-Naphthoquinone | ||
File:Naphthoquinone_reaction_with_butadiene.tif|Reaction of 1,4-Naphthoquinone with butadiene | File:Naphthoquinone_reaction_with_butadiene.tif|Reaction of 1,4-Naphthoquinone with butadiene | ||
</gallery> | |||
<gallery caption="1,4-Naphthoquinone"> | |||
File:1,4-Naphthoquinone.svg|1,4-Naphthoquinone | |||
File:1,4-Naphthoquinone-3D-balls.png|1,4-Naphthoquinone 3D model | |||
File:Naphthoquinone_reaction_with_butadiene.tif|Naphthoquinone reaction with butadiene | |||
</gallery> | </gallery> | ||
Latest revision as of 04:00, 18 February 2025
1,4-Naphthoquinone[edit]


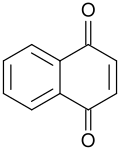
1,4-Naphthoquinone is an organic compound with the formula C__H_O_. It is a yellow crystalline solid that is a derivative of naphthalene. It is one of the two isomeric naphthoquinones, the other being 1,2-naphthoquinone.
Structure and Properties[edit]
1,4-Naphthoquinone consists of a naphthalene ring with two ketone groups at the 1 and 4 positions. This structure imparts a planar geometry to the molecule, which is typical of quinones. The compound is characterized by its yellow color and its ability to undergo redox reactions, making it a useful intermediate in various chemical processes.
Synthesis[edit]
1,4-Naphthoquinone can be synthesized through the oxidation of naphthalene using various oxidizing agents such as chromic acid or potassium permanganate. Another method involves the oxidation of 1,4-dihydroxynaphthalene.
Reactions[edit]
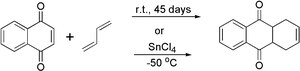
1,4-Naphthoquinone is a versatile compound in organic synthesis. It can undergo Diels-Alder reactions with dienes, such as butadiene, to form adducts. This reaction is useful in the synthesis of complex organic molecules.
Applications[edit]
1,4-Naphthoquinone is used in the production of dyes, vitamin K analogs, and as a precursor to various pharmaceuticals. Its ability to participate in redox reactions makes it valuable in the study of electron transfer processes.
Safety[edit]
1,4-Naphthoquinone is a hazardous substance and should be handled with care. It can cause irritation to the skin, eyes, and respiratory tract. Proper safety precautions, including the use of personal protective equipment, should be observed when handling this compound.
Related Compounds[edit]
Related Pages[edit]
-
Structural formula of 1,4-Naphthoquinone
-
3D ball-and-stick model of 1,4-Naphthoquinone
-
Reaction of 1,4-Naphthoquinone with butadiene
- 1,4-Naphthoquinone
-
1,4-Naphthoquinone
-
1,4-Naphthoquinone 3D model
-
Naphthoquinone reaction with butadiene