Phorbol: Difference between revisions
CSV import |
CSV import |
||
| Line 15: | Line 15: | ||
{{chemistry-stub}} | {{chemistry-stub}} | ||
<gallery> | |||
File:Phorbol.svg|Phorbol chemical structure | |||
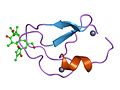
File:PDB_1ptr_EBI.jpg|Phorbol binding in protein structure | |||
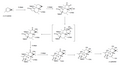
File:Phorbol_synthesis.png|Phorbol synthesis pathway | |||
</gallery> | |||
Latest revision as of 02:12, 18 February 2025

Phorbol is a natural, organic compound derived from the seeds of the Croton tiglium, a plant belonging to the Euphorbiaceae family. Phorbols are known for their role as the active component in croton oil, which has been used in traditional medicine for its potent biological activities. The structure of phorbol is characterized by a tetracyclic diterpene backbone, which is crucial for its biological functions and interactions with cellular mechanisms.
The significance of phorbol extends into the field of biochemistry and molecular biology, particularly in the study of protein kinase C (PKC) activation. Phorbol esters, which are derivatives of phorbol, mimic diacylglycerol (DAG), a physiological activator of PKC. This mimicry allows phorbol esters to bind to and activate PKC, leading to a variety of cellular responses. Due to this property, phorbol esters are widely used as research tools to investigate the signaling pathways mediated by PKC and its role in processes such as cell growth, differentiation, and apoptosis.
However, the potent biological activity of phorbol esters also associates them with toxic and carcinogenic effects. Exposure to high levels of phorbol esters can lead to skin irritation, inflammation, and even skin cancer in laboratory animals, highlighting the importance of understanding their mechanisms of action and potential health impacts.
In the medical and pharmaceutical fields, research on phorbol and its derivatives continues to explore their therapeutic potential. Their ability to modulate PKC activity suggests possible applications in treating diseases where PKC is implicated, such as cancer, Alzheimer's disease, and psoriasis. However, the challenge lies in harnessing their biological activities while minimizing toxicity.
-
Phorbol chemical structure
-
Phorbol binding in protein structure
-
Phorbol synthesis pathway