Aminorex: Difference between revisions
CSV import Tags: mobile edit mobile web edit |
CSV import |
||
| Line 30: | Line 30: | ||
{{medicine-stub}} | {{medicine-stub}} | ||
<gallery> | |||
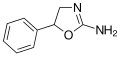
File:Aminorex_structure.svg|Chemical structure of Aminorex | |||
File:Aminorex_molecule_ball.png|Ball-and-stick model of Aminorex | |||
File:Aminorex_rxn_mech.png|Reaction mechanism of Aminorex | |||
</gallery> | |||
Latest revision as of 01:12, 18 February 2025
Aminorex is a stimulant that is derived from amphetamine. It was first synthesized in the 1960s and was used as an appetite suppressant in the treatment of obesity. However, it was later withdrawn from the market due to its potential for abuse and the serious health risks associated with its use.
History[edit]
Aminorex was first synthesized in 1962 by McNeil Laboratories. It was marketed as an appetite suppressant under the brand name Menocil. However, it was withdrawn from the market in the 1970s due to concerns about its potential for abuse and the serious health risks associated with its use.
Pharmacology[edit]
Aminorex acts as a norepinephrine-dopamine releasing agent (NDRA). It stimulates the release of these neurotransmitters in the brain, which results in increased alertness, energy, and appetite suppression. However, it also has the potential to cause serious health problems, including pulmonary hypertension and cardiovascular disease.
Side Effects[edit]
The side effects of aminorex can be severe and potentially life-threatening. They include hypertension, tachycardia, insomnia, anxiety, and psychosis. Long-term use can also lead to dependence and withdrawal symptoms.
Legal Status[edit]
Aminorex is classified as a Schedule I controlled substance in the United States, meaning it has a high potential for abuse and no accepted medical use. It is also controlled under international law by the United Nations Convention on Psychotropic Substances.
See Also[edit]
-
Chemical structure of Aminorex
-
Ball-and-stick model of Aminorex
-
Reaction mechanism of Aminorex