Tetrahydroxy-1,4-benzoquinone: Difference between revisions
CSV import Tags: mobile edit mobile web edit |
CSV import Tags: mobile edit mobile web edit |
||
| Line 32: | Line 32: | ||
{{chemistry-stub}} | {{chemistry-stub}} | ||
{{medicine-stub}} | {{medicine-stub}} | ||
<gallery> | |||
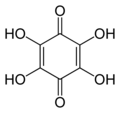
File:Tetrahydroxy-1,4-benzoquinone-2D-skeletal.png|2D skeletal structure of Tetrahydroxy-1,4-benzoquinone | |||
</gallery> | |||
Latest revision as of 02:06, 17 February 2025
Tetrahydroxy-1,4-benzoquinone is a type of quinone, a class of organic compounds that are formally "derived from aromatic compounds [such as benzene or naphthalene] by conversion of an even number of –CH= groups into –C(=O)– groups with any necessary rearrangement of double bonds", resulting in "a fully conjugated cyclic dione structure". The term quinone is also used more broadly to refer to the class of compounds that are formally derived from quinones through replacement of one or more of their carbonyl groups with other functional groups.
Structure and properties[edit]
Tetrahydroxy-1,4-benzoquinone, also known as hydroquinone, is a type of benzoquinone characterized by the presence of four hydroxyl groups. It has the chemical formula C6H4(OH)4. The molecule is planar, and the OH groups are oriented in the same direction. This gives the molecule a polar nature, which affects its solubility and reactivity.
Synthesis[edit]
Tetrahydroxy-1,4-benzoquinone can be synthesized from benzene through a series of reactions involving oxidation and hydroxylation. The process typically involves the use of strong oxidizing agents and acidic conditions.
Applications[edit]
Tetrahydroxy-1,4-benzoquinone is used in various industrial applications due to its redox properties. It is used as a reducing agent in photography and as a developer in the production of black and white films. It is also used in the manufacture of dyes and pigments, and as a polymerization inhibitor.
Health effects[edit]
Exposure to tetrahydroxy-1,4-benzoquinone can cause skin and eye irritation. Ingestion or inhalation can lead to more serious health effects, including damage to the respiratory and digestive systems.
See also[edit]
References[edit]
<references />
-
2D skeletal structure of Tetrahydroxy-1,4-benzoquinone