2,5-Dimethoxy-4-(2-fluoroethyl)amphetamine: Difference between revisions
CSV import Tags: mobile edit mobile web edit |
CSV import |
||
| Line 39: | Line 39: | ||
[[Category:Serotonin receptor agonists]] | [[Category:Serotonin receptor agonists]] | ||
[[Category:Substituted amphetamines]] | [[Category:Substituted amphetamines]] | ||
== 2,5-Dimethoxy-4-(2-fluoroethyl)amphetamine == | |||
<gallery> | |||
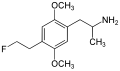
File:2,5-Dimethoxy-4-(2-fluoroethyl)amphetamine.svg|Chemical structure of 2,5-Dimethoxy-4-(2-fluoroethyl)amphetamine | |||
</gallery> | |||
Latest revision as of 01:46, 17 February 2025
2,5-Dimethoxy-4-(2-fluoroethyl)amphetamine[edit]

2,5-Dimethoxy-4-(2-fluoroethyl)amphetamine (also known as DOEF) is a psychedelic amphetamine of the substituted amphetamine class. It is a derivative of the phenethylamine 2C-E, with a fluorine atom replacing the ethyl group at the 4-position of the aromatic ring.
Chemical Structure[edit]
2,5-Dimethoxy-4-(2-fluoroethyl)amphetamine is a member of the substituted amphetamines, which are characterized by the presence of a phenethylamine core with an alpha-methyl group. The chemical structure of DOEF includes two methoxy groups at the 2 and 5 positions of the aromatic ring, and a 2-fluoroethyl group at the 4 position.
Pharmacology[edit]
DOEF acts as a serotonin receptor agonist, primarily affecting the 5-HT2A receptor. This action is responsible for its psychedelic effects. The compound is structurally similar to other psychedelic amphetamines such as DOM and DOI, which are known for their potent hallucinogenic properties.
Effects[edit]
The effects of 2,5-Dimethoxy-4-(2-fluoroethyl)amphetamine are similar to those of other psychedelic amphetamines. Users report altered states of consciousness, visual and auditory hallucinations, and changes in perception of time and space. The intensity and duration of these effects can vary depending on the dose and individual sensitivity.
Synthesis[edit]
The synthesis of DOEF involves the introduction of a 2-fluoroethyl group to the 4-position of the aromatic ring of 2,5-dimethoxyamphetamine. This is typically achieved through a series of chemical reactions starting from 2,5-dimethoxybenzaldehyde.
Legal Status[edit]
The legal status of 2,5-Dimethoxy-4-(2-fluoroethyl)amphetamine varies by country. In many jurisdictions, it is considered a controlled substance due to its structural similarity to other regulated psychedelic compounds.
Related Compounds[edit]
- 2,5-Dimethoxy-4-ethylamphetamine (DOET)
- 2,5-Dimethoxy-4-iodoamphetamine (DOI)
- 2,5-Dimethoxy-4-methylamphetamine (DOM)
- 2C-E
See Also[edit]
Related Pages[edit]
2,5-Dimethoxy-4-(2-fluoroethyl)amphetamine[edit]
-
Chemical structure of 2,5-Dimethoxy-4-(2-fluoroethyl)amphetamine