Brilanestrant: Difference between revisions
CSV import |
CSV import Tags: mobile edit mobile web edit |
||
| Line 1: | Line 1: | ||
'''Brilanestrant''' is a | {{Short description|Overview of the drug Brilanestrant}} | ||
{{Drugbox | |||
| image = Brilanestrant.svg | |||
| image_size = 250px | |||
| image_alt = Chemical structure of Brilanestrant | |||
}} | |||
'''Brilanestrant''' is a nonsteroidal [[selective estrogen receptor degrader]] (SERD) that has been investigated for its potential use in the treatment of [[breast cancer]]. It functions by binding to the [[estrogen receptor]] and promoting its degradation, thereby inhibiting the growth of estrogen receptor-positive cancer cells. | |||
==Mechanism of Action== | ==Mechanism of Action== | ||
Brilanestrant | Brilanestrant acts as a selective estrogen receptor degrader. Unlike [[selective estrogen receptor modulators]] (SERMs), which modulate the receptor's activity, SERDs like Brilanestrant lead to the degradation of the receptor itself. This is achieved through the recruitment of [[ubiquitin ligase]] complexes that tag the receptor for destruction by the [[proteasome]]. By reducing the number of estrogen receptors available, Brilanestrant effectively diminishes the proliferative signaling in estrogen receptor-positive [[tumors]]. | ||
==Clinical | ==Clinical Development== | ||
Brilanestrant has been evaluated in | Brilanestrant has been evaluated in several [[clinical trials]] to assess its efficacy and safety in patients with [[hormone receptor-positive breast cancer]]. These trials have explored its use both as a monotherapy and in combination with other [[anticancer agents]]. The drug has shown promise in preclinical models and early-phase clinical studies, demonstrating the ability to reduce tumor size and delay disease progression. | ||
== | ==Pharmacokinetics== | ||
The | The pharmacokinetic profile of Brilanestrant includes its absorption, distribution, metabolism, and excretion. It is administered orally and has been shown to have a favorable bioavailability. The drug is metabolized primarily in the [[liver]] and excreted through the [[urinary]] and [[biliary]] systems. Understanding the pharmacokinetics of Brilanestrant is crucial for optimizing dosing regimens and minimizing potential side effects. | ||
== | ==Side Effects== | ||
As with many anticancer therapies, Brilanestrant may cause a range of side effects. Commonly reported adverse effects include [[nausea]], [[fatigue]], and [[hot flashes]]. More serious side effects, although less common, can include [[liver toxicity]] and [[thromboembolic events]]. Monitoring and managing these side effects is an important aspect of patient care during treatment with Brilanestrant. | |||
== | ==Future Directions== | ||
Brilanestrant | Research into Brilanestrant continues, with ongoing studies aimed at better understanding its role in the treatment of breast cancer. There is interest in exploring its use in combination with other targeted therapies, such as [[CDK4/6 inhibitors]] and [[PI3K inhibitors]], to enhance its therapeutic efficacy. Additionally, studies are investigating its potential application in other estrogen receptor-positive cancers. | ||
[[ | ==Related pages== | ||
[[ | * [[Selective estrogen receptor degrader]] | ||
[[ | * [[Breast cancer]] | ||
[[ | * [[Estrogen receptor]] | ||
* [[Hormone therapy (oncology)]] | |||
[[Category:Antineoplastic drugs]] | |||
[[Category:Selective estrogen receptor degraders]] | |||
Revision as of 10:49, 15 February 2025
Overview of the drug Brilanestrant
| Brilanestrant | |
|---|---|
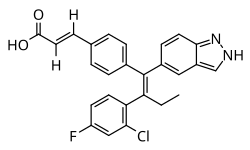
| |
| INN | |
| Drug class | |
| Routes of administration | |
| Pregnancy category | |
| Bioavailability | |
| Metabolism | |
| Elimination half-life | |
| Excretion | |
| Legal status | |
| CAS Number | |
| PubChem | |
| DrugBank | |
| ChemSpider | |
| KEGG | |
Brilanestrant is a nonsteroidal selective estrogen receptor degrader (SERD) that has been investigated for its potential use in the treatment of breast cancer. It functions by binding to the estrogen receptor and promoting its degradation, thereby inhibiting the growth of estrogen receptor-positive cancer cells.
Mechanism of Action
Brilanestrant acts as a selective estrogen receptor degrader. Unlike selective estrogen receptor modulators (SERMs), which modulate the receptor's activity, SERDs like Brilanestrant lead to the degradation of the receptor itself. This is achieved through the recruitment of ubiquitin ligase complexes that tag the receptor for destruction by the proteasome. By reducing the number of estrogen receptors available, Brilanestrant effectively diminishes the proliferative signaling in estrogen receptor-positive tumors.
Clinical Development
Brilanestrant has been evaluated in several clinical trials to assess its efficacy and safety in patients with hormone receptor-positive breast cancer. These trials have explored its use both as a monotherapy and in combination with other anticancer agents. The drug has shown promise in preclinical models and early-phase clinical studies, demonstrating the ability to reduce tumor size and delay disease progression.
Pharmacokinetics
The pharmacokinetic profile of Brilanestrant includes its absorption, distribution, metabolism, and excretion. It is administered orally and has been shown to have a favorable bioavailability. The drug is metabolized primarily in the liver and excreted through the urinary and biliary systems. Understanding the pharmacokinetics of Brilanestrant is crucial for optimizing dosing regimens and minimizing potential side effects.
Side Effects
As with many anticancer therapies, Brilanestrant may cause a range of side effects. Commonly reported adverse effects include nausea, fatigue, and hot flashes. More serious side effects, although less common, can include liver toxicity and thromboembolic events. Monitoring and managing these side effects is an important aspect of patient care during treatment with Brilanestrant.
Future Directions
Research into Brilanestrant continues, with ongoing studies aimed at better understanding its role in the treatment of breast cancer. There is interest in exploring its use in combination with other targeted therapies, such as CDK4/6 inhibitors and PI3K inhibitors, to enhance its therapeutic efficacy. Additionally, studies are investigating its potential application in other estrogen receptor-positive cancers.