Water: Difference between revisions
CSV import Tag: Replaced |
No edit summary |
||
| Line 1: | Line 1: | ||
[[File:Water_molecule_(1).svg|thumb|right|200px|The structure of a water molecule.]] | [[File:Water_molecule_(1).svg|thumb|right|200px|The structure of a water molecule.]] | ||
| Line 36: | Line 34: | ||
== Distribution and Cycle == | == Distribution and Cycle == | ||
Water covers about 71% of the Earth's surface, mostly in [[oceans]] and other large bodies of water. It is also found in [[rivers]], [[lakes]], [[glaciers]], and [[groundwater]]. | Water covers about 71% of the Earth's surface, mostly in [[oceans]] and other large bodies of water. It is also found in [[rivers]], [[lakes]], [[glaciers]], and [[groundwater]]. | ||
Latest revision as of 19:02, 21 February 2025

Water is a transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms. It is vital for all known forms of life, even though it provides no calories or organic nutrients.
Chemical and Physical Properties[edit]

Water is a chemical compound with the chemical formula H₂O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a polar molecule with an electrical dipole moment. It is also capable of forming hydrogen bonds, which are responsible for many of its unique properties.
States of Matter[edit]

Water exists in three states of matter: solid, liquid, and gas. In its solid state, it is known as ice. In its gaseous state, it is known as water vapor or steam. The transition between these states occurs at specific temperatures and pressures.
Phase Diagram[edit]
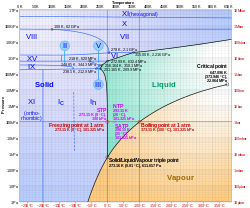
The phase diagram of water shows the conditions of temperature and pressure under which water exists in different states. The diagram includes the triple point and critical point of water, which are important in understanding its behavior under various conditions.
Hydrogen Bonding[edit]
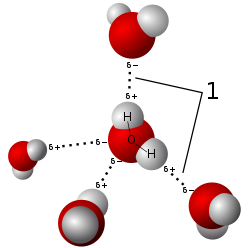
Hydrogen bonding is a significant factor in the properties of water. These bonds occur between the hydrogen atom of one water molecule and the oxygen atom of another, leading to a high level of cohesion and surface tension.
Heat Capacity[edit]

Water has a high specific heat capacity, meaning it can absorb a lot of heat before it begins to get hot. This property makes water an excellent coolant and helps regulate the Earth's climate.
Distribution and Cycle[edit]
Water covers about 71% of the Earth's surface, mostly in oceans and other large bodies of water. It is also found in rivers, lakes, glaciers, and groundwater.
Water Cycle[edit]

The water cycle describes the continuous movement of water on, above, and below the surface of the Earth. It involves processes such as evaporation, condensation, precipitation, and infiltration.
Importance to Life[edit]
Water is essential for all known forms of life. It acts as a solvent, a temperature buffer, a metabolite, and a living environment. It is involved in many biological processes, including photosynthesis, respiration, and digestion.
Related Pages[edit]
Lua error: bad argument #2 to 'title.new' (unrecognized namespace name 'Portal').