Aconitase: Difference between revisions
CSV import |
CSV import |
||
| Line 9: | Line 9: | ||
File:Isocitrate_Zoom_Final.png|Isocitrate binding site | File:Isocitrate_Zoom_Final.png|Isocitrate binding site | ||
</gallery> | </gallery> | ||
== Aconitase == | |||
'''Aconitase''' is an enzyme that plays a crucial role in the [[citric acid cycle]], also known as the [[Krebs cycle]], which is a key metabolic pathway that provides energy in the form of [[adenosine triphosphate]] (ATP) through the oxidation of [[acetyl-CoA]] derived from carbohydrates, fats, and proteins. Aconitase catalyzes the stereospecific isomerization of [[citrate]] to [[isocitrate]] via the intermediate [[cis-aconitate]]. | |||
== Structure == | |||
Aconitase is an [[iron-sulfur protein]] that contains a [4Fe-4S] cluster, which is essential for its enzymatic activity. The enzyme exists in two forms: mitochondrial aconitase, which is involved in the citric acid cycle, and cytosolic aconitase, which also functions as an [[iron regulatory protein]] (IRP1) that regulates [[iron metabolism]] by binding to [[iron-responsive elements]] (IREs) on [[mRNA]]. | |||
== Function == | |||
In the citric acid cycle, aconitase catalyzes the reversible isomerization of citrate to isocitrate. This reaction involves the dehydration of citrate to form cis-aconitate, followed by the rehydration of cis-aconitate to produce isocitrate. This step is crucial for the continuation of the citric acid cycle, as isocitrate is subsequently oxidized by [[isocitrate dehydrogenase]] to produce [[_-ketoglutarate]], [[carbon dioxide]], and [[NADH]]. | |||
== Mechanism == | |||
The mechanism of aconitase involves the coordination of the substrate citrate to the [4Fe-4S] cluster, which facilitates the removal of a hydroxyl group from citrate to form cis-aconitate. The enzyme then re-adds a hydroxyl group to the intermediate, resulting in the formation of isocitrate. The [4Fe-4S] cluster plays a critical role in the enzyme's ability to catalyze this reaction by stabilizing the transition state and facilitating the rearrangement of the substrate. | |||
== Regulation == | |||
Aconitase activity is regulated by the availability of its [4Fe-4S] cluster, which can be affected by cellular iron levels and oxidative stress. In conditions of low iron availability or oxidative stress, the [4Fe-4S] cluster can be disassembled, converting aconitase into its inactive form. In its cytosolic form, aconitase acts as an iron regulatory protein, binding to IREs on mRNA to regulate the translation of proteins involved in iron storage and transport. | |||
== Clinical Significance == | |||
Dysfunction of aconitase can lead to metabolic disorders due to impaired energy production. Additionally, the enzyme's role in iron metabolism links it to conditions such as [[iron deficiency anemia]] and [[hemochromatosis]]. The sensitivity of the [4Fe-4S] cluster to oxidative damage also implicates aconitase in [[neurodegenerative diseases]] such as [[Parkinson's disease]], where oxidative stress is a contributing factor. | |||
== Related pages == | |||
* [[Citric acid cycle]] | |||
* [[Iron-sulfur protein]] | |||
* [[Isocitrate dehydrogenase]] | |||
* [[Iron regulatory protein]] | |||
{{Enzymes}} | |||
{{Citric acid cycle}} | |||
[[Category:Enzymes]] | |||
[[Category:Iron-sulfur proteins]] | |||
[[Category:Metabolism]] | |||
Latest revision as of 00:35, 19 February 2025
-
Aconitase structure
-
Aconitase enzyme
-
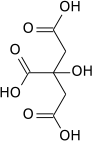
Citrate molecule
-
Cis-Aconitate molecule
-
Isocitric acid structure
-
Aconitase reaction mechanism
-
Citrate binding site
-
Isocitrate binding site
Aconitase[edit]
Aconitase is an enzyme that plays a crucial role in the citric acid cycle, also known as the Krebs cycle, which is a key metabolic pathway that provides energy in the form of adenosine triphosphate (ATP) through the oxidation of acetyl-CoA derived from carbohydrates, fats, and proteins. Aconitase catalyzes the stereospecific isomerization of citrate to isocitrate via the intermediate cis-aconitate.
Structure[edit]
Aconitase is an iron-sulfur protein that contains a [4Fe-4S] cluster, which is essential for its enzymatic activity. The enzyme exists in two forms: mitochondrial aconitase, which is involved in the citric acid cycle, and cytosolic aconitase, which also functions as an iron regulatory protein (IRP1) that regulates iron metabolism by binding to iron-responsive elements (IREs) on mRNA.
Function[edit]
In the citric acid cycle, aconitase catalyzes the reversible isomerization of citrate to isocitrate. This reaction involves the dehydration of citrate to form cis-aconitate, followed by the rehydration of cis-aconitate to produce isocitrate. This step is crucial for the continuation of the citric acid cycle, as isocitrate is subsequently oxidized by isocitrate dehydrogenase to produce _-ketoglutarate, carbon dioxide, and NADH.
Mechanism[edit]
The mechanism of aconitase involves the coordination of the substrate citrate to the [4Fe-4S] cluster, which facilitates the removal of a hydroxyl group from citrate to form cis-aconitate. The enzyme then re-adds a hydroxyl group to the intermediate, resulting in the formation of isocitrate. The [4Fe-4S] cluster plays a critical role in the enzyme's ability to catalyze this reaction by stabilizing the transition state and facilitating the rearrangement of the substrate.
Regulation[edit]
Aconitase activity is regulated by the availability of its [4Fe-4S] cluster, which can be affected by cellular iron levels and oxidative stress. In conditions of low iron availability or oxidative stress, the [4Fe-4S] cluster can be disassembled, converting aconitase into its inactive form. In its cytosolic form, aconitase acts as an iron regulatory protein, binding to IREs on mRNA to regulate the translation of proteins involved in iron storage and transport.
Clinical Significance[edit]
Dysfunction of aconitase can lead to metabolic disorders due to impaired energy production. Additionally, the enzyme's role in iron metabolism links it to conditions such as iron deficiency anemia and hemochromatosis. The sensitivity of the [4Fe-4S] cluster to oxidative damage also implicates aconitase in neurodegenerative diseases such as Parkinson's disease, where oxidative stress is a contributing factor.
Related pages[edit]
| Enzymes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|