Dimerization: Difference between revisions
CSV import |
CSV import |
||
| Line 41: | Line 41: | ||
[[Category:Biochemistry]] | [[Category:Biochemistry]] | ||
{{chemistry-stub}} | {{chemistry-stub}} | ||
<gallery> | |||
File:Carboxylic_acid_dimers.png|Carboxylic acid dimers | |||
File:Dicyclopentadiene_structure.svg|Dicyclopentadiene structure | |||
File:Borane_&_Diborane.jpg|Borane and Diborane | |||
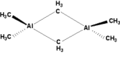
File:Trimethylaluminium_dimer.png|Trimethylaluminium dimer | |||
File:Tubulin_dimer.png|Tubulin dimer | |||
File:Receptor_Tyrosine_Kinase_Dimerization.jpg|Receptor Tyrosine Kinase Dimerization | |||
</gallery> | |||
Latest revision as of 10:58, 18 February 2025
Dimerization is a chemical process where two molecules, often identical, combine to form a larger complex known as a dimer. This process is significant in various fields of chemistry, biochemistry, and molecular biology, influencing the behavior and function of many compounds and proteins.
Overview[edit]
Dimerization involves the interaction of two monomer units. These units can be simple molecules, such as organic compounds, or more complex structures like proteins. The forces that drive dimerization include hydrogen bonds, ionic bonds, van der Waals forces, and covalent bonds. The specific nature of the interaction depends on the properties of the molecules involved.
Types of Dimerization[edit]
Dimerization can occur in several forms, depending on the nature of the molecules and the conditions under which they interact:
Homodimerization[edit]
In homodimerization, two identical molecules join to form a dimer. This is common in proteins where two identical subunits form a functional complex, as seen in many enzymes and receptors.
Heterodimerization[edit]
Heterodimerization involves the pairing of two different molecules. This type is crucial in cellular signaling and regulatory mechanisms, particularly in the formation of receptor complexes in cell membranes.
Covalent Dimerization[edit]
This form involves the formation of a covalent bond between two monomers. An example is the dimerization of alkenes through photochemistry or radical mechanisms.
Non-Covalent Dimerization[edit]
Non-covalent dimerization does not involve covalent bonds but rather electrostatic, hydrogen bonding, or hydrophobic interactions. This type is typical in the dimerization of nucleic acids and some proteins.
Biological Significance[edit]
In biology, dimerization is crucial for the function of many proteins and enzymes. It can affect the enzyme kinetics, substrate specificity, and regulatory mechanisms of proteins. For example, the dimerization of receptor tyrosine kinases is essential for signal transduction and cellular response to external signals.
Applications[edit]
Dimerization has practical applications in various industries:
- In pharmaceuticals, understanding protein dimerization can lead to the development of drugs that inhibit or promote this process, impacting disease treatment.
- In polymer chemistry, dimerization is a step in creating larger polymer structures, affecting the properties and applications of synthetic materials.
Challenges and Research[edit]
Research in dimerization continues to address challenges such as controlling the specificity and stability of dimers in therapeutic and industrial applications. Advanced techniques in molecular biology and structural biology are used to study and manipulate dimerization processes.
See Also[edit]
-
Carboxylic acid dimers
-
Dicyclopentadiene structure
-
Borane and Diborane
-
Trimethylaluminium dimer
-
Tubulin dimer
-
Receptor Tyrosine Kinase Dimerization