Dipropanoylmorphine: Difference between revisions
CSV import Tags: mobile edit mobile web edit |
CSV import |
||
| Line 1: | Line 1: | ||
{{Short description|A semi-synthetic opioid derived from morphine}} | |||
{{Drugbox | |||
| verifiedfields = changed | |||
| verifiedrevid = 477002123 | |||
| image = Dipropanoylmorphine.svg | |||
| image_size = 200px | |||
| IUPAC_name = 3,6-Dipropanoyloxy-4,5-epoxymorphinan-7-one | |||
| CAS_number = 8002-20-2 | |||
}} | |||
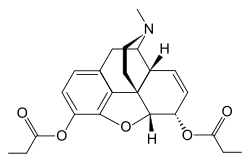
'''Dipropanoylmorphine''' is a [[semi-synthetic opioid]] derived from [[morphine]]. It is an ester of morphine, specifically the 3,6-dipropionate ester. This compound is part of a class of drugs known as [[opioid analgesics]], which are used for their pain-relieving properties. | |||
Dipropanoylmorphine is a morphine | |||
==Chemical Structure and Properties== | |||
[[File:Dipropanoylmorphine.svg|thumb|right|Chemical structure of Dipropanoylmorphine]] | |||
Dipropanoylmorphine is chemically similar to morphine, with the addition of two propionyl groups at the 3 and 6 positions of the morphine molecule. This modification alters its pharmacokinetic properties, potentially affecting its potency, duration of action, and solubility compared to morphine. | |||
==Pharmacology== | ==Pharmacology== | ||
Dipropanoylmorphine acts primarily | Dipropanoylmorphine acts on the [[opioid receptors]] in the [[central nervous system]], primarily the [[mu-opioid receptor]]. This interaction leads to the inhibition of [[neurotransmitter]] release, resulting in analgesia, sedation, and euphoria. Like other opioids, it can also cause respiratory depression, constipation, and physical dependence. | ||
==Medical Use== | ==Medical Use== | ||
While dipropanoylmorphine has been studied for its analgesic properties, it is not commonly used in clinical practice. The development of more effective and safer opioid analgesics has limited its use. However, it remains of interest in research settings for understanding the structure-activity relationships of opioid compounds. | |||
== | ==Synthesis== | ||
The synthesis of dipropanoylmorphine involves the esterification of morphine with propionic anhydride. This chemical reaction results in the formation of the dipropionate ester, which is dipropanoylmorphine. The process requires careful control of reaction conditions to ensure the desired product is obtained. | |||
== | ==Legal Status== | ||
Dipropanoylmorphine is classified as a controlled substance in many jurisdictions due to its potential for abuse and addiction. Its legal status is similar to that of other opioids, and it is subject to strict regulations regarding its manufacture, distribution, and use. | |||
==Related pages== | |||
* [[Morphine]] | * [[Morphine]] | ||
* [[Opioid]] | * [[Opioid]] | ||
* [[Opioid receptor]] | |||
* [[Analgesic]] | * [[Analgesic]] | ||
[[Category:Opioids]] | [[Category:Opioids]] | ||
[[Category: | [[Category:Semi-synthetic opioids]] | ||
[[Category: | [[Category:Phenanthrenes]] | ||
Latest revision as of 05:11, 16 February 2025
A semi-synthetic opioid derived from morphine
| Dipropanoylmorphine | |
|---|---|
| |
| INN | |
| Drug class | |
| Routes of administration | |
| Pregnancy category | |
| Bioavailability | |
| Metabolism | |
| Elimination half-life | |
| Excretion | |
| Legal status | |
| CAS Number | 8002-20-2 |
| PubChem | |
| DrugBank | |
| ChemSpider | |
| KEGG | |
Dipropanoylmorphine is a semi-synthetic opioid derived from morphine. It is an ester of morphine, specifically the 3,6-dipropionate ester. This compound is part of a class of drugs known as opioid analgesics, which are used for their pain-relieving properties.
Chemical Structure and Properties[edit]

Dipropanoylmorphine is chemically similar to morphine, with the addition of two propionyl groups at the 3 and 6 positions of the morphine molecule. This modification alters its pharmacokinetic properties, potentially affecting its potency, duration of action, and solubility compared to morphine.
Pharmacology[edit]
Dipropanoylmorphine acts on the opioid receptors in the central nervous system, primarily the mu-opioid receptor. This interaction leads to the inhibition of neurotransmitter release, resulting in analgesia, sedation, and euphoria. Like other opioids, it can also cause respiratory depression, constipation, and physical dependence.
Medical Use[edit]
While dipropanoylmorphine has been studied for its analgesic properties, it is not commonly used in clinical practice. The development of more effective and safer opioid analgesics has limited its use. However, it remains of interest in research settings for understanding the structure-activity relationships of opioid compounds.
Synthesis[edit]
The synthesis of dipropanoylmorphine involves the esterification of morphine with propionic anhydride. This chemical reaction results in the formation of the dipropionate ester, which is dipropanoylmorphine. The process requires careful control of reaction conditions to ensure the desired product is obtained.
Legal Status[edit]
Dipropanoylmorphine is classified as a controlled substance in many jurisdictions due to its potential for abuse and addiction. Its legal status is similar to that of other opioids, and it is subject to strict regulations regarding its manufacture, distribution, and use.