Pyrrole: Difference between revisions
CSV import |
CSV import Tags: mobile edit mobile web edit |
||
| Line 33: | Line 33: | ||
{{stub}} | {{stub}} | ||
== Pyrrole == | |||
<gallery> | |||
File:Pyrrole-2D-full.svg|Pyrrole 2D full | |||
File:Pyrrole-2D-numbered.svg|Pyrrole 2D numbered | |||
File:Pyrrole-CRC-MW-3D-balls-A.png|Pyrrole CRC MW 3D balls A | |||
File:Pyrrole-CRC-MW-3D-vdW.png|Pyrrole CRC MW 3D vdW | |||
File:Heme_B.svg|Heme B | |||
File:Pyrrolsynthese1.svg|Pyrrolsynthese 1 | |||
File:Hantzsch_Pyrrole_Synthesis_Scheme.png|Hantzsch Pyrrole Synthesis Scheme | |||
File:Knorr_Pyrrole_Synthesis_Scheme.png|Knorr Pyrrole Synthesis Scheme | |||
File:Paal-Knorr_Pyrrole_Synthesis.svg|Paal-Knorr Pyrrole Synthesis | |||
File:Van_Leusen_Mechanism.jpg|Van Leusen Mechanism | |||
File:Barton-Zard_reaction.svg|Barton-Zard reaction | |||
File:Piloty-Robinson_reaction.png|Piloty-Robinson reaction | |||
</gallery> | |||
Latest revision as of 21:29, 23 February 2025
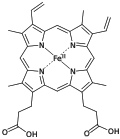
Pyrrole is a heterocyclic aromatic organic compound, a five-membered ring with the formula C4H4NH. It is a colorless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., N-methylpyrrole, C4H4NCH3. Porphobilinogen, a trisubstituted pyrrole, is the biosynthetic precursor to many natural products such as heme.
Structure and bonding[edit]
Pyrroles are aromatic. Like furan and thiophene, the pyrrole ring is aromatic because it contains 4 π-electrons in the two double bonds plus one lone pair of electrons on the nitrogen. The lone pair on the nitrogen is not part of the aromatic π-system. Pyrroles are weakly basic, with a conjugate acid pKa of −3.8. The most basic site is C-2. Pyrroles are also weakly acidic at the N-H position, with a pKa of 17.5.
Preparation[edit]
Pyrrole can be prepared by treatment of furan with ammonia in the presence of acid catalysts, such as AlCl3. Pyrrole can also be formed by dehydrogenation of pyrrolidine.
Reactions[edit]
Pyrrole undergoes reactions that are characteristic of other aromatic compounds, notably electrophilic aromatic substitution. It is particularly susceptible to such reactions because of the electron-donating effect of the nitrogen. Hence, electrophiles attack pyrrole at the 2 position, then rearrangement of the intermediate σ-complex leads to substitution at the 3 position.
Applications[edit]
Pyrrole is a constituent of tobacco smoke and not as an ingredient. Pyrrole is used as a precursor to the drug tolmetin. Polypyrrole is of some commercial value.
See also[edit]
References[edit]
<references />
|
|
|
Pyrrole[edit]
-
Pyrrole 2D full
-
Pyrrole 2D numbered
-
Pyrrole CRC MW 3D balls A
-
Pyrrole CRC MW 3D vdW
-
Heme B
-
Pyrrolsynthese 1
-
Hantzsch Pyrrole Synthesis Scheme
-
Knorr Pyrrole Synthesis Scheme
-
Paal-Knorr Pyrrole Synthesis
-
Van Leusen Mechanism
-
Barton-Zard reaction
-
Piloty-Robinson reaction