Triarylmethane dye: Difference between revisions
CSV import Tags: mobile edit mobile web edit |
CSV import |
||
| Line 1: | Line 1: | ||
{{Short description|Class of synthetic dyes derived from triphenylmethane}} | |||
{{Use dmy dates|date=October 2023}} | |||
'''Triarylmethane dyes''' are a class of synthetic dyes derived from [[triphenylmethane]]. These dyes are characterized by their vivid colors and are used in a variety of applications, including as [[pH indicator]]s, [[biological stain]]s, and [[textile dye]]s. They are known for their intense coloration and ability to bind to various substrates. | |||
== Properties | ==Structure and Properties== | ||
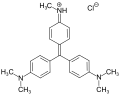
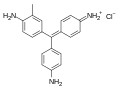
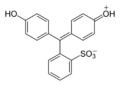
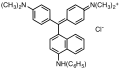
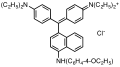
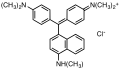
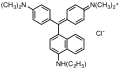
Triarylmethane dyes are | Triarylmethane dyes are based on the [[triphenylmethane]] structure, which consists of three [[aromatic ring]]s attached to a central carbon atom. The basic structure can be modified by introducing different substituents on the aromatic rings, leading to a wide range of colors and properties. These dyes are typically cationic and can form salts with anions, which enhances their solubility in water. | ||
== | ==Applications== | ||
Triarylmethane dyes have a variety of applications due to their bright colors and ability to bind to different materials. Some common uses include: | |||
* '''Textile Industry''': These dyes are used to color fabrics and textiles due to their vibrant hues and ability to adhere to fibers. | |||
* '''Biological Staining''': In [[histology]] and [[microbiology]], triarylmethane dyes are used to stain cells and tissues, aiding in the visualization of cellular structures under a microscope. | |||
* '''pH Indicators''': Certain triarylmethane dyes change color in response to pH changes, making them useful as indicators in [[titration]]s and other chemical analyses. | |||
== | ==Examples of Triarylmethane Dyes== | ||
Some well-known triarylmethane dyes include: | |||
* [[Methyl violet]] | |||
* [[Crystal violet]] | |||
* [[Malachite green]] | |||
* [[Bromocresol green]] | |||
* [[Phenolphthalein]] | |||
== | ==Safety and Environmental Impact== | ||
While triarylmethane dyes are useful in many applications, they can pose environmental and health risks. Some of these dyes are toxic and can cause skin irritation or allergic reactions. Additionally, their persistence in the environment can lead to pollution of water bodies, affecting aquatic life. | |||
==Related Pages== | |||
* [[Dye]] | * [[Dye]] | ||
* [[ | * [[Synthetic dye]] | ||
* [[ | * [[Azo dye]] | ||
* [[ | * [[Anthraquinone dye]] | ||
==References== | |||
{{Reflist}} | |||
==Gallery== | |||
<gallery> | |||
File:Methyl_Violet_2B.svg|Methyl Violet 2B | |||
File:Methyl_Violet_6B.svg|Methyl Violet 6B | |||
File:Kristallviolett.svg|Crystal Violet | |||
File:Pararosaniline.png|Pararosaniline | |||
File:Rosaniline_hydrochloride.svg|Rosaniline Hydrochloride | |||
File:Phenolphthalein-low-pH-2D-skeletal.svg|Phenolphthalein | |||
File:Phenol-red-zwitterionic-form-2D-skeletal.png|Phenol Red | |||
File:Chlorophenol_red.png|Chlorophenol Red | |||
File:Cresol_Red.svg|Cresol Red | |||
File:Bromocresol_purple.svg|Bromocresol Purple | |||
File:Bromocresol_green.svg|Bromocresol Green | |||
File:Malachite_green_structure.svg|Malachite Green | |||
File:Structure_of_the_dye_brilliant_green.png|Brilliant Green | |||
File:Brilliant_Blue_FCF(2).svg|Brilliant Blue FCF | |||
File:Victoria_blue_B_with_charge.svg|Victoria Blue B | |||
File:Victoria_blue_FBR_revised.svg|Victoria Blue FBR | |||
File:Victoria_blue_BO.svg|Victoria Blue BO | |||
File:Victoria_pure_blue_FGA_revised.svg|Victoria Pure Blue FGA | |||
File:Victoria_blue_4_R_revised.svg|Victoria Blue 4R | |||
File:Victoria_blue_R_revised.svg|Victoria Blue R | |||
File:EosinB.png|Eosin B | |||
File:EosinY.png|Eosin Y | |||
File:rhodamine_B.svg|Rhodamine B | |||
File:Rhodamine_123.svg|Rhodamine 123 | |||
File:Fluorescein_2.svg|Fluorescein | |||
File:ThymolphthaleinSynthesis.png|Thymolphthalein | |||
File:Bromocresol_green_ionic_equilibrium.png|Bromocresol Green Ionic Equilibrium | |||
</gallery> | |||
[[Category:Triarylmethane dyes]] | |||
Revision as of 01:19, 10 February 2025
Class of synthetic dyes derived from triphenylmethane
Triarylmethane dyes are a class of synthetic dyes derived from triphenylmethane. These dyes are characterized by their vivid colors and are used in a variety of applications, including as pH indicators, biological stains, and textile dyes. They are known for their intense coloration and ability to bind to various substrates.
Structure and Properties
Triarylmethane dyes are based on the triphenylmethane structure, which consists of three aromatic rings attached to a central carbon atom. The basic structure can be modified by introducing different substituents on the aromatic rings, leading to a wide range of colors and properties. These dyes are typically cationic and can form salts with anions, which enhances their solubility in water.
Applications
Triarylmethane dyes have a variety of applications due to their bright colors and ability to bind to different materials. Some common uses include:
- Textile Industry: These dyes are used to color fabrics and textiles due to their vibrant hues and ability to adhere to fibers.
- Biological Staining: In histology and microbiology, triarylmethane dyes are used to stain cells and tissues, aiding in the visualization of cellular structures under a microscope.
- pH Indicators: Certain triarylmethane dyes change color in response to pH changes, making them useful as indicators in titrations and other chemical analyses.
Examples of Triarylmethane Dyes
Some well-known triarylmethane dyes include:
Safety and Environmental Impact
While triarylmethane dyes are useful in many applications, they can pose environmental and health risks. Some of these dyes are toxic and can cause skin irritation or allergic reactions. Additionally, their persistence in the environment can lead to pollution of water bodies, affecting aquatic life.
Related Pages
References
<references group="" responsive="1"></references>
Gallery
-
Methyl Violet 2B
-
Methyl Violet 6B
-
Crystal Violet
-
Pararosaniline
-
Rosaniline Hydrochloride
-
Phenolphthalein
-
Phenol Red
-
Chlorophenol Red
-
Cresol Red
-
Bromocresol Purple
-
Bromocresol Green
-
Malachite Green
-
Brilliant Green
-
Brilliant Blue FCF
-
Victoria Blue B
-
Victoria Blue FBR
-
Victoria Blue BO
-
Victoria Pure Blue FGA
-
Victoria Blue 4R
-
Victoria Blue R
-
Eosin B
-
Eosin Y
-
Rhodamine B
-
Rhodamine 123
-
Fluorescein
-
Thymolphthalein
-
Bromocresol Green Ionic Equilibrium