Acetoacetic acid: Difference between revisions
CSV import |
CSV import Tags: mobile edit mobile web edit |
||
| Line 55: | Line 55: | ||
[[Category:Metabolism]] | [[Category:Metabolism]] | ||
[[Category:Organic chemistry]] | [[Category:Organic chemistry]] | ||
== Acetoacetic_acid == | |||
<gallery> | |||
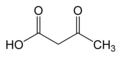
File:Acetoacetic_acid.png|Acetoacetic acid structure | |||
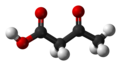
File:Acetoacetic-acid-3D-balls.png|3D model of acetoacetic acid | |||
File:3-Oxobutyric_acid_KetoEnol.svg|Keto-enol tautomerism of acetoacetic acid | |||
File:YellowPig5thTry.png|Acetoacetic acid | |||
File:DiketeneReaction.svg|Reaction involving diketene and acetoacetic acid | |||
</gallery> | |||
Latest revision as of 04:34, 18 February 2025
Acetoacetic acid[edit]

Acetoacetic acid is a beta-keto acid with the chemical formula C4H6O3. It is an important intermediate in the ketone bodies metabolism and is involved in various biochemical pathways.
Structure and Properties[edit]

Acetoacetic acid is a keto acid that contains both a ketone group and a carboxylic acid group. It exists in equilibrium with its enol form, which is a characteristic feature of beta-keto acids. The compound is a colorless liquid with a pungent odor and is soluble in water.
Keto-Enol Tautomerism[edit]

Acetoacetic acid exhibits keto-enol tautomerism, where the keto form (3-oxobutyric acid) and the enol form are in dynamic equilibrium. This tautomerism is important in its reactivity and stability.
Biological Role[edit]
Acetoacetic acid is one of the three ketone bodies produced in the liver during the breakdown of fatty acids. It serves as an important energy source during periods of fasting, prolonged exercise, or low-carbohydrate diets. The other ketone bodies are beta-hydroxybutyrate and acetone.
Synthesis[edit]
Acetoacetic acid can be synthesized through the Claisen condensation of ethyl acetate with acetone. It is also produced in the body from the breakdown of fatty acids in the liver.
Reactions[edit]
Acetoacetic acid is a versatile intermediate in organic synthesis. It can undergo decarboxylation to form acetone and carbon dioxide. It can also participate in various condensation reactions, such as the Diketene reaction.

Uses[edit]
Acetoacetic acid and its esters are used in the synthesis of dyes, pharmaceuticals, and flavoring agents. Its derivatives are also used in the production of vitamins and hormones.
Safety[edit]
Acetoacetic acid is a corrosive substance and should be handled with care. It can cause irritation to the skin, eyes, and respiratory tract.
Related pages[edit]
Gallery[edit]
-
Illustration of acetoacetic acid
Acetoacetic_acid[edit]
-
Acetoacetic acid structure
-
3D model of acetoacetic acid
-
Keto-enol tautomerism of acetoacetic acid
-
Acetoacetic acid
-
Reaction involving diketene and acetoacetic acid