AP endonuclease: Difference between revisions
CSV import |
CSV import |
||
| Line 37: | Line 37: | ||
[[Category:DNA repair enzymes]] | [[Category:DNA repair enzymes]] | ||
[[Category:Molecular biology]] | [[Category:Molecular biology]] | ||
<gallery> | |||
File:ApEndonuclease1.jpg|AP endonuclease structure | |||
File:APE1DNABend.jpg|APE1 DNA bending | |||
File:APE1activelabeled.JPG|APE1 active site labeled | |||
File:MechanismAPE1colour_2.svg|Mechanism of APE1 | |||
File:Lucanthone.png|Lucanthone | |||
</gallery> | |||
Latest revision as of 04:57, 18 February 2025
AP Endonuclease[edit]

AP endonuclease is a crucial enzyme involved in the DNA repair process, specifically in the base excision repair (BER) pathway. It plays a vital role in maintaining the integrity of the genome by repairing abasic sites, which are sites in DNA that have lost a nucleotide base.
Function[edit]
AP endonucleases recognize and cleave the phosphodiester backbone at abasic sites, creating a nick in the DNA strand. This action is essential for the removal of damaged bases and the subsequent repair of the DNA strand. The enzyme's activity is critical for preventing mutations and maintaining genomic stability.
Mechanism[edit]

The mechanism of AP endonuclease involves the recognition of an abasic site followed by the cleavage of the DNA backbone. The enzyme uses a metal ion-dependent mechanism to hydrolyze the phosphodiester bond, generating a 3'-hydroxyl group and a 5'-deoxyribose phosphate group. This cleavage allows for the removal of the damaged site and the subsequent filling in of the gap by DNA polymerase and DNA ligase.
Types[edit]
There are several types of AP endonucleases, with APE1 (also known as APEX1) being the most well-studied in humans. APE1 is a multifunctional protein that not only acts as an AP endonuclease but also has roles in RNA processing and transcriptional regulation.
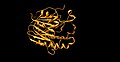
Structure[edit]

AP endonucleases typically have a conserved structure that allows them to interact with DNA and recognize abasic sites. The structure of APE1, for example, reveals a DNA-binding domain that induces a bend in the DNA, facilitating the recognition and cleavage of the abasic site.
Clinical Significance[edit]
AP endonucleases are important for preventing genetic disorders and cancer by repairing DNA damage. Mutations or deficiencies in these enzymes can lead to increased susceptibility to mutagenesis and disease. Inhibitors of AP endonucleases, such as Lucanthone, are being studied for their potential use in cancer therapy by sensitizing cancer cells to chemotherapy and radiation.

Related Pages[edit]
Gallery[edit]
-
Active site of APE1 labeled.
-
AP endonuclease structure
-
APE1 DNA bending
-
APE1 active site labeled
-
Mechanism of APE1
-
Lucanthone