Sonogashira coupling: Difference between revisions
CSV import Tags: mobile edit mobile web edit |
CSV import |
||
| Line 22: | Line 22: | ||
[[Category:Carbon-carbon bond forming reactions]] | [[Category:Carbon-carbon bond forming reactions]] | ||
{{chemistry-stub}} | {{chemistry-stub}} | ||
<gallery> | |||
File:Sonogashira_reaction_scheme_ACS.png|Sonogashira coupling | |||
File:Sonogashira-reaction-mechanism.png|Sonogashira coupling | |||
File:Cu-free-mechanism.png|Sonogashira coupling | |||
File:Reactivity_in_Sonogashira_Cross-Coupling.svg|Sonogashira coupling | |||
File:Symmetrical_sonogashira_1-bromo-4-iodobenzene.png|Sonogashira coupling | |||
File:Fe-catalysed-Sonogashira.png|Sonogashira coupling | |||
File:Polymeric_phosphine_ligand.png|Sonogashira coupling | |||
File:Najera,_Org_Lett_2003.png|Sonogashira coupling | |||
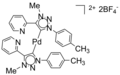
File:Pd-NHC-1.png|Sonogashira coupling | |||
File:PyNHC-PEPPSI-water.png|Sonogashira coupling | |||
File:Alkynylation.svg|Sonogashira coupling | |||
File:Bulgaramine.png|Sonogashira coupling | |||
</gallery> | |||
Latest revision as of 11:44, 18 February 2025

The Sonogashira coupling is a chemical reaction used in organic chemistry for the formation of carbon-carbon bonds, specifically between an alkyne and an aryl or vinyl halide using a palladium catalyst, often in conjunction with a copper co-catalyst, and a base. This reaction is highly valuable for the construction of complex organic molecules, including pharmaceuticals, natural products, and polymers.
The reaction was first reported in 1975 by Kenkichi Sonogashira and Nobue Hagihara. It has since become a staple in the toolbox of synthetic organic chemists due to its reliability, versatility, and the mild conditions under which it can be performed.
Mechanism[edit]
The Sonogashira coupling involves several key steps. Initially, the palladium catalyst coordinates to the aryl or vinyl halide, forming a palladium-halide complex. This complex undergoes oxidative addition, inserting the palladium into the carbon-halide bond. Subsequently, a transmetalation step occurs where the alkyne, activated by the copper co-catalyst, exchanges its copper partner for palladium. Finally, reductive elimination releases the coupled product and regenerates the palladium catalyst.
Conditions[edit]
Typical conditions for the Sonogashira coupling include the use of a palladium catalyst, such as Pd(PPh3)4, and a copper(I) salt, like CuI, in the presence of a base, such as triethylamine or diisopropylamine. The reaction is often performed under an inert atmosphere of nitrogen or argon to prevent oxidation of the sensitive components. Solvents commonly used include dimethylformamide (DMF), toluene, and dichloromethane (DCM).
Applications[edit]
The Sonogashira coupling has found widespread application in the synthesis of complex organic molecules. It is particularly useful in the construction of conjugated systems, such as those found in organic light-emitting diodes (OLEDs), pharmaceuticals, and agrochemicals. Its ability to form carbon-carbon bonds efficiently and under mild conditions makes it a preferred method for modifying sensitive molecules.
Variations[edit]
Several variations of the Sonogashira coupling have been developed to improve its efficiency, broaden its substrate scope, or reduce its environmental impact. These include the use of water as a solvent, microwave-assisted reactions, and the development of ligand-free conditions. Additionally, efforts have been made to replace palladium with more abundant and less expensive metals, though palladium remains the most commonly used catalyst.
Safety and Environmental Considerations[edit]
While the Sonogashira coupling is a powerful tool in organic synthesis, it does involve the use of toxic heavy metals and potentially hazardous conditions. Proper safety precautions should be taken, including the use of an inert atmosphere and the proper disposal of palladium and copper residues. Recent research has focused on making the reaction more environmentally friendly by minimizing waste and avoiding the use of toxic solvents and reagents.
-
Sonogashira coupling
-
Sonogashira coupling
-
Sonogashira coupling
-
Sonogashira coupling
-
Sonogashira coupling
-
Sonogashira coupling
-
Sonogashira coupling
-
Sonogashira coupling
-
Sonogashira coupling
-
Sonogashira coupling
-
Sonogashira coupling
-
Sonogashira coupling