Diphenylmethylpiperazine: Difference between revisions
CSV import |
CSV import |
||
| Line 25: | Line 25: | ||
{{Chem-stub}} | {{Chem-stub}} | ||
{{medicine-stub}} | {{medicine-stub}} | ||
<gallery> | |||
File:Diphenylmethylpiperazine.svg|Diphenylmethylpiperazine structure | |||
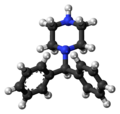
File:Diphenylmethylpiperazine_3D_ball.png|Diphenylmethylpiperazine 3D ball model | |||
</gallery> | |||
Latest revision as of 01:14, 18 February 2025
Diphenylmethylpiperazine (DMP) is a chemical compound that belongs to the class of organic compounds known as piperazines. Piperazines are compounds containing a piperazine ring, which is a six-membered ring with two nitrogen atoms and four carbon atoms.
Chemical Structure[edit]
Diphenylmethylpiperazine is composed of a piperazine ring attached to a diphenylmethyl group. The diphenylmethyl group consists of a methyl group (CH3) attached to two phenyl groups. The chemical formula of diphenylmethylpiperazine is C17H20N2.
Synthesis[edit]
The synthesis of diphenylmethylpiperazine involves the reaction of diphenylmethyl chloride with piperazine in the presence of a base. This reaction is typically carried out in a polar solvent such as dimethylformamide (DMF).
Applications[edit]
Diphenylmethylpiperazine has been used in the synthesis of various pharmaceutical drugs. It is a key intermediate in the production of antidepressants and antipsychotics. It is also used in the synthesis of antihistamines and antiemetics.
Safety[edit]
As with all chemical compounds, handling diphenylmethylpiperazine requires appropriate safety measures. It may cause irritation to the skin and eyes, and may be harmful if swallowed or inhaled. Always use personal protective equipment and work in a well-ventilated area when handling this compound.
See Also[edit]
-
Diphenylmethylpiperazine structure
-
Diphenylmethylpiperazine 3D ball model